DrEddyClinic.com -You will find here reliable information's about unconventional, unorthodox, unproven, or alternative, complementary, innovative, integrative therapies and western traditional medicine as well.
Monday, April 27, 2009
Chewing Gum Releases Mercury From Dental Fillings
Thursday, September 18, 2008
Amalgam free Dentistry
There is much discussion about the toxic content of this filling material. At St Leonards Holistic Dental Care, we believe it is an outdated material that may be affecting your health. Amalgam fillings can be removed safely when the correct precautions are taken. Newer, more advanced tooth coloured materials can be used to replace mercury amalgam. Continue Reading >>
Chemical & Heavy Metal Cleanse Starter Kit$234.75  The Chemical & Heavy Metal Starter Kit was prepared by Dr. Group for the person who is new to the cleansing process, or for the person who is simply looking for an easy-to-perform, cost effective cleanse program. The Starter Kit includes: 1-Bottle of Zeotrex™ (30-Day supply), 1 Mineral Check® Testing Kit and 3-Packages of Dr. Group's Detox Foot Pads (15-Day Supply) |
more discussion: Forum
· Addiction Forum · Ask the Doctors Forum · Ayurveda Forum · Ayurvedic & Thai Herbs Forum · Colon Cleansing Forum · Dental Forum · Diabetes Forum · Diet Forum · General Cleansing Forum · Hepatitis A, B. C Forum · Integrated Medicine Forum · Live Blood Analysis Forum · Ozone-Oxygen-Forum · pH - Alkaline - Acidity Forum · Weight Loss Forum
Monday, August 18, 2008
Natural Dental Health
 “The terms oral health and general health should not be interpreted as separate entities. Oral health is integral to general health: oral health means more than healthy teeth and you cannot be healthy without oral health.” —Donna Shalala, Secretary of Health and Human Services, in Oral Health America: A Report of the Surgeon General, 2000
“The terms oral health and general health should not be interpreted as separate entities. Oral health is integral to general health: oral health means more than healthy teeth and you cannot be healthy without oral health.” —Donna Shalala, Secretary of Health and Human Services, in Oral Health America: A Report of the Surgeon General, 2000The renouned German physician Dr. Reinhard Voll estimated that nearly 80% of all illness is related entirely or partially to problems in the mouth. The reason the teeth are such a threat to health is that, in addition to their connection to every organ and gland in the body, they can harbor infections without symptoms. There's no pain or discomfort. Yet, there may be chronic infection eroding the body's immune response-wearing out the immune system. This infection is very difficult to detect. Few people today have escaped the problems of dental cavities and gum infection. About 98% of Americans have some areas of diseased gum tissue in their mouths, over half of these are also experiencing a progressive "bone loss." Fortunately, cavities and pyorrhea (gum disease and bone loss) are both 100% preventable and reversible.
The mouth is a hostile environment. It's warm, moist, and full of nutritient-laden saliva, decaying teeth, and soggy gums, which makes it a haven for bacteria. Teeth are subject to sudden changes of temperature created by extremes such as coffee and ice cream. Mechanical stresses challenge the mouth in the form of a combination of hard and soft foods. It is attacked chemically by foods that are highly acidic and highly alkaline with overtones of salinity and sugar. All these conditions provide corrosive influences, necessitating artificial replacements supplied by the dentist.
Saturday, August 16, 2008
Mercury Detoxification Procedures: Amalgam Fillings
Mercury is linked to the most degenerative diseases known to man. In many cases these diseases are iatrogenic - i.e. diseases caused by inappropriate medical / dental treatment.
Mercury is the second most toxic metal known to man; second only to plutonium. But regardless of ranking, mercury has proven to be the most devastating to the health of mankind through its ability to induce diverse, degenerative diseases. In terms of its toll on human suffering, no other metal comes close. Many common medical and mental problems are known to be linked to, if not caused by mercury toxicity.
The majority of mercury that accumulates in our bodies comes from the "silver" amalgam fillings in our teeth. But mercury can be picked up from numerous other sources including artificial flowers, ammunition cartridges, various chemicals including aldehydes, acetic acid, acetone (as in finger nail polish removers), alcohols (as in spirits and rubbing alcohols), chlorine (as in drinking water and chlorine bleaches), disinfectants, cosmetics, Q-tips, dental appliances, dary cell batteries, dyes, electroplated products, felt hats, gold, inks, vapor lamps, mirrors, pharmaceutical products (drugs), pottery, prints, radio tubes, storage batteries, thermometers, wood preservatives, and others. Continue Reading >>
Chemical & Heavy Metal Cleanse Starter Kit$149.85  The Chemical & Heavy Metal Starter Kit was designed by Dr. Group for individuals that are new to the cleansing process, or are simply looking for an easy-to-perform, cost effective cleanse program. The Heavy Metal Starter Kit is comprised of LIFE Detox Foot Patches™, NDF Plus™, and Quantum Zeolite™. |
Friday, August 08, 2008
Holistic Oral Care
Holistic dentistry promotes preventative oral care and maintenance, and an environmental commitment to reducing waste and using non-toxic materials.
The holistic dentist eschews the use of mercury and silver-based amalgam fillings and traditional x-rays, which create waste chromium and require other hazardous processing chemicals, in lieu of safe, biocompatible filling materials and digital imaging. Continue Reading >>
Chemical & Heavy Metal Cleanse Starter Kit$149.85  The Chemical & Heavy Metal Starter Kit was designed by Dr. Group for individuals that are new to the cleansing process, or are simply looking for an easy-to-perform, cost effective cleanse program. The Heavy Metal Starter Kit is comprised of LIFE Detox Foot Patches™, NDF Plus™, and Quantum Zeolite™. |
Tuesday, July 01, 2008
 Here are a few samples of toxicity reactions to mercury/amalgam dental fillings and/or improvements after removing the fillings.
Here are a few samples of toxicity reactions to mercury/amalgam dental fillings and/or improvements after removing the fillings.I found these post and email on the Internet. The names have beenremoved to protect confidentiality, but many of these posts can befound in various archives and WWW search engines.
Please not that this is only a small percentage of adverse reactions to mercury amalgams that have been posted to the Internet over the last several months. The adverse reactions posted to the Internet are only avery small percentage of the total adverse reactions reported to various groups. There are no official lists of adverse reactions as far as I know. Please join the AMALGAM mailing list and see the dental links and files on my home page for more information.
Adverse Reaction Samples From the Internet
Further scientific information can be found at Mercury Adverse Effects Web Page, 150 Year's of Russian Roulette Web Page, Alt Corp's Amalgam Page, and Bo Walhjalt's Mercury Articles Web Page. More information about removal, detoxification, and placement of composite fillings can be found at Bioprobe, Inc. and at the Preventive Dental Association.
more information about amalgam and toxins: http://www.dreddyclinic.com/integrated_med/amalgammercurydentalfilling.htm
Saturday, March 15, 2008
Amalgam Removals
Thefillings were a very large part of the problem and if I hadn't hadthem removed I know that I would not be alive today. So, I am verythankful to the "quacks" such as a kinesiologist/nutritionist, an MD"environmental medicine (ecology)" specialist, and a licensednaturopath who helped me with the diagnosis and recovery.
Saturday, January 06, 2007
Amalgam / Mercury Dental Filling Toxicity
 An often overlooked, but extremely important source of toxic material is the mercury from silver [mercury] amalgam fillings. Some people who are aware of the situation are confused by the mixture of information available. Unfortunately, statements from dental trade organizations and on a few poorly-researched news reports have muddled the situation.
An often overlooked, but extremely important source of toxic material is the mercury from silver [mercury] amalgam fillings. Some people who are aware of the situation are confused by the mixture of information available. Unfortunately, statements from dental trade organizations and on a few poorly-researched news reports have muddled the situation.Here are a few facts about mercury amalgam fillings:
Causes Damage to Brain in ChildrenIn February, 1998, a group of the world's top mercury researchers announced that mercury from amalgam fillings can permanently damage the brain, kidneys, and immune system of children.
Amalgam Fillings Linked to Neurological Problems, Gastrointestinal Problems
The first large-scale epidemiological study of mercury and adverse reactions was recently completed and showed that of the symptoms looked at, there was a link seen to gastrointestinal problems, sleep disturbances, concentration problems, memory disturbances, lack of initiative, restlessness, bleeding gums and other mouth disorders.
Mercury / Alzheimer's Disease Connection Found
A study related to mercury and Alzheimer's Disease was recently completed by a team of scientists led by well-respected researcher Dr. Boyd Haley. They exposed rats to levels of mercury vapor diluted to account for size differences between humans and rats. The rats developed tissue damage "indistinguishable" from that of Alzheimer's Disease.
Repeating the experiment showed the same results. Dr. Haley is quoted as saying "I'm getting the rest of my fillings taken out right now, and I've asked my wife to have hers replaced too." Also see: http://www.holistic-dentistry.com/artalzeimer.asp
Amalgam Fillings Since 1970s Unstable
The type of mercury fillings that began to be used during the last couple of decades, non-gamma-2 (high copper), releases many times more mercury than the older style of amalgam fillings. Also, please see the article on the instability of dental amalgam fillings by Ulf Bengsston.
Amalgam Fillings Release Highly Toxic Elemental MercuryMercury is one of the most toxic substances known. The mercury release from fillings is absorbed primarily as highly toxic elemental mercury vapor.
Amalgam Fillings Largest Source of Mercury
By FarBased on a number of studies in Sweden, the World Health Organization review of inorganic mercury in 1991 determined that mercury absorption is estimated to be approximately four times higher from amalgam fillings than from fish consumption. Recent studies have confirmed this estimate. The amount absorbed can vary considerably from person to person.
Gold Crowns, Gum, Bruxism, Computer Monitors Increase Release of Mercury Significantly
Gum chewing, grinding of teeth/bruxism, computer terminal exposure, presence of gold fillings or gold crowns (even if covering mercury fillings), teeth brushing, braces, and chewing cause the release of significantly increased amounts of mercury from the fillings.
Also, please see the following short review related to increases in mercury exposure from dissimilar metals in the mouth, exposure to magnetic fields, chewing, etc.
Cumulative Poison and Builds Up in OrgansMercury released from fillings builds up in the brain, pituitary, adrenals, and other parts of the body.
Mercury Amalgam Fillings Effect Porphyrins
Preliminary results from the first detailed biochemical analysis (scroll half-way down) of patients who removed mercury amalgam fillings showed a significant drop in the excretion of porphyrins (important to heme synthesis -- heme carries oxygen to red blood cells), as well as a number of other key biochemical changes. Also, see the Video of the preliminary results from the study.
Potential Contribuatory Factor in Other Diseases
Mercury from amalgam fillings has been implicated as a possible contribuatory factor in some cases of multiple sclerosis, Parkinson's Disease, IBS, reproductive disorders, allergies, and a variety of other illnesses.
Mercury Build Up in Brain, Organs and Breast Milk of Fetuses of Mothers With Amalgam FillingsMercury from fillings in pregnant women has been shown to cause mercury accumulation in brain, kidneys and liver of human fetuses (all of the areas tested). Studies have shown that mercury can be passed to infants from breast milk.
Proper Removal of Fillings Produces Eventual Health Improvement
A recent study published in the Journal of Orthomolecular Medicine related to the proper removal of mercury amalgam fillings from 118 subjects showed an elimination or reduction or 80% of the classic mercury poisoning symptoms. In many cases, it took 6 to 12 months after mercury amalgam removal for the symptoms to disappear.
World-reknowned Experts Agree About Potential Danger
In contrast to statements from dental trade organizations, toxicologists and medical researchers are often quite concerned about the use of mercury. Lars Friberg, the lead toxicologist on the World Health Organization team looking at inorganic mercury and health effects recently stated that he believes that mercury is unsuitable for dental materials because of safety concerns.
Canadian Class Action LawsuitCanadians are in the process of beginning a major class action lawsuit based on the fact that the government knew of but did not warn the public about mercury dangers from amalgam fillings. Legal actions related to mercury exposure from mercury amalgam fillings and vaccines are beginning in the United States. For more information and a directory of Mercury-free dentists, please see the TalkInternation.com web site.
Obviously, not everyone experiences acute toxicity effects from the mercury in amalgam fillings. However, virtually everyone does have mercury build up in their bodies from implantation of such fillings. The large increase in mercury exposure from the newer non-gamma-2 mercury fillings means that only time will tell how much damage has been caused by daily exposure to mercury to such fillings.I do not recommend that people assume automatically that they will be healed by the removal of amalgam fillings.
Many people are helped tremendously and some are healed. The 80% figure for people showing improvement within a year likely refers to people who had good reason to suspect that they were being significantly effected by the fillings. The percentage of people in the general population who might experience health improvement within one year after removal is probably much lower than 80%.
I recommend going into the mercury amalgam removal procedure knowing that, at the very least, you will have removed yourself from a regular exposure to an extremely toxic material such that it will not build up in your organs and possibly cause significant health problems at a later date.Mercury amalgam fillings should be removed only by dentists with experience using the IOAMT mercury amalgam removal protocol (presented with the permission of the excellent Preventive Dental Association web page).
Such dentists are often experienced with proper evaluation and placement of composite fillings, both of which can be crucial for the success of the treatment. Biocompatability tests are often important in determining which composite materials can be safely used. I believe that composite (plastic) fillings are a better replacement than metal (e.g., gold) fillings even in chemically-sensitive individuals.
They are, however, not without safety questions, but are still likely to be much less toxic than mercury amalgam fillings. Proper placement of composites should be left to experienced amalgam removal dentists as the average well-meaning dentist may not be aware of the newer placement techniques.
Further scientific information can be found at Mercury Adverse Effects Web Page, 150 Year's of Russian Roulette Web Page, Alt Corp's Amalgam Page, and Bo Walhjalt's Mercury Articles Web Page. More information about removal, detoxification, and placement of composite fillings can be found at Bioprobe, Inc. and at the Preventive Dental Association. Information about finding a dentist practicing non-toxic dentistry can be found on the Resources For Related to Non-Toxic Dentistry web page.
Also, the AMALGAM mailing list can be a good source of accurate, up-to-date information.
Important Links
- Alt Corp Amalgam Web Page (Scientific links on left of page)
- Adverse Reaction Samples From the Internet - Updated 7/01/96
- Amalgam-Free Dentists (T.E.S.T.)
- Amalgam-Free Dentists (Talk International)
- Bikerchick Amalgam Poisoning web page
- Bio-Probe, Inc. -- Scientific/General/Detox Book & Product Catalog
- Blazing Tattles 3-Part Series on Cleaning Up Dental Work
- Canadians For Mercury Relief (CFMR)
- Chronic Illness and Chronic Mercury Exposure -- Dr. Edelson's Page
- Clifford Consulting & Research (Dental Materials Compatibility Testing)
- Consumers for Dental Choice
- Dental amalgam - 150 years of Russian Roulette (Dagfinn Reiers¿l)
- Hal A. Huggins, DDS, MS - Research In Toxicity
- Mercury/Amalgam Fillings-Related Illness FAQ
- Vimy Dental Group Web page
- Preventive Dental Health Association
Saturday, December 23, 2006
AMALGAM REMOVAL - Correct & Incorrect Protocols
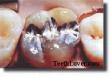 International Academy of Oral Medicine and ToxicologyProtocol for Mercury/Silver Filling Removal[1]
International Academy of Oral Medicine and ToxicologyProtocol for Mercury/Silver Filling Removal[1]International Academy of Oral Medicine and Toxicology
Protocol for Mercury/Silver Filling Removal[1]
Patient protection:
First in every concerned doctor's mind is the protection of the patient from additional exposure to mercury. This is especially true ofthe mercury-toxic patient. The mercury-toxic patient may have been exposed to varying amounts of mercury from diet, environment, employment or from mercury/silver dental fillings.
All forms are cumulative and can contribute to the body burden. The goal of this preferred procedure is to minimize any additional exposure of the patient, ourselves, or staff to mercury.
During chewing the patient is exposed to intraoral levels which areseveral times the EPA allowable air concentration.[2] During the removal or placement of amalgam the patient can be exposed to amounts which are a thousand times greater than the EPA allowable concentration.[3] Once the drill touches the filling, temperature increases, immediately vaporizing the mercury component of the alloy. There are 8 steps to greatly reducing everyone's exposure.
Step one Keep the fillings cool
1) All removal must be done under cold water spray with copious amounts of water. Once the removal has begun, the mercury vapour will be continuously released from the tooth.
2) Therefore, A high volume evacuator tip should be kept near the tooth (1/2 inch) at all times to evacuate this vapour from the area of the patient. Polishing amalgam can create very dangerous levels of mercury and should be avoided especially for the mercury toxic patient.
3) All patients having amalgam removed or placed should be provided with an alternative air source and instructed to not breathe through their mouth during treatment. A nasal hood such as is used with the nitrous oxide analgesia equipment is excellent.
Air is best and oxygen is acceptable although not required. If just air is used it should be clean and free of mercury vapour preferably from outside the dental office.
4) Particles of mercury alloy should be washed and vacuumed away as soon as they are generated. The filling should be sectioned and removed in large pieces to reduce exposure. At present the International Academy of Oral Medicine and Toxicology (IAOMT) has approved removal both with and without the use of a rubber dam.
Some evidence exists to support both views since high levels of mercury and amalgam particles can be found under the dam. All members are agreed that whether or not a rubber dam is used, the patient should be instructed to not breathe through their mouth or swallow the particles.
Some experts feel that it is better to remove the amalgam first and then apply the dam, if needed, for restorative procedures.
5) After the fillings have been removed, take off the rubber dam if one was used and lavage the patients mouth for at least 30 seconds with cold water and vacuum. Remove your gloves and replace them with a new pair. If a restorative procedure is next then reapply a new dam and proceed.
6) Immediately change patients protective wear and clean their face.
7) Consider appropriate nutritional support before, during and after removal.
8) Install room air purifiers or ionizers and fans for everyone's well being.
Staff protection OSHA [4,5] requires that employees be given written informed consent before the use of any toxic chemicals, of which mercury is one. Elemental mercury vapor is one of the most toxic forms of mercury and should not breathed. Women of child bearing age should be exposed to no more than 10% of the OSHA MAC [6]. Women who are pregnant should be exposed to no mercury.[7] If you use mercury or remove mercury in any form, the National Institute of Occupational Safety and Health (NIOSH) has recommended that your employees be medically monitored annually.
9) Any mercury exposure requires that the employee wear an approved mercury filter mask. An approved mask is appropriate for wearing during all dental procedures which will expose you or your staff to mercury.[8]
The manner in which dentists operate their equipment dramaticallyaffects the amount of mercury released. Never drill on mercury high dry.
It is hazardous to you, your staff, and your patient. Levels as high as4000 mg/M3 have been measured 18" from the drill when using high dry. Levels over 1000 mg/M3 are measurable upon opening an amalgam mixing capsule.
One out of 7 Californian dental offices tested over the OSHA TWA safety limit of 50 mg/M3. 100% of the vacuum cleaner exhaust tested over 100 mg/M3. Any office where mercury is used should be tested regularly and staff should be monitored for exposure. Testing services are available and a mercury sensor badge is available for personnel monitoring. They should test inside storage areas and along baseboards, where mercury might have dropped. Office spills can go undetected for years and are extremely hazardous.
REFERENCES[1] IAOMT Standards of Care Preferred Procedure Approved 9/27/92[2] EPA United States Environmental Protection Agency Office of Health and Environment Assessment Mercury health effects update Final Report EPA-600/8-84-019F 1971 EPA[3] Cooley RL, Barkmeier WW: Mercury vapour emitted during ultraspeed cutting of amalgam. J Indiana Dent Assoc 57:28-31, 1978[4] OSHA Job Health Series: Mercury.(2234)8/1975[5] Hazard Communication Program Federal Register/ Vol. 52. No. 163 / Monday, August 24, 1987[6] OSHA MAC is Threshold Limit Value of 100 micrograms/ cubic meter or 100 PPM This is a never to be exceeded standard.[7] Koos BJ and Lango LD , Mercury Toxicity in the pregnant woman, foetus, and newborn infant. A review Am J Obstetrics and Gynaecology, 126(3):390-409, 1976[8] Mine Safety Association high levels and 3M mercury dust mask lower levelsa) Patient Preparation for Amalgam Removal
AMALGAM REMOVAL PREPARATION WARNING: When the body is exposed to amalgam mercury it has an on-going need for detoxification and healing processes. If you have a medical condition, then hormones and enzymes the body needs to heal have likely been depleted by this on-going detoxification and healing process.
So before your amalgam restorations are removed, blood testing should be performed to determine what hormones and enzymes are deficient. Based on the blood test results a medical doctor can evaluate what nutritional and hormonal supplements are needed to prepare the body. After amalgams are removed, the healing usually accelerates, so there will be an even greater demand for the hormones and enzymes that were depleted. So a patient with a medical condition should take nutritional and hormonal supplements before, during and after amalgam removal.
b) Dental Procedures for Patient Protection During Amalgam Removal
IAOMT Standards of Care, Preferred Procedure, "Reducing Mercury Vapor Exposure for the Patient During Amalgam Removal." (September 1992)
The IAOMT has currently established the following amalgam removal protocols. If these protocols are followed, the amount of mercury released into the body during amalgam removal is reduced.
Place a rubber dam around the tooth to isolate it from the body.
Provide an alternative source of air to the patient.
Place a saliva ejector under the dam to remove mercury vapour that penetrates the latex.
Use high volume evacuation with isolate attachment.
Section amalgams and remove in as large pieces as possible.
Remove and properly dispose of rubber dam and mercury after amalgam removal.
Other amalgam removal precautions in addition to the protocols listed above include:
Remove no more than two amalgams per appointment.
Time amalgam removal appointments at least one month apart.
Administer intravenous Vitamin C before removal (Hg has a greater affinity to Vitamin C that is present in the blood than it does for body tissue).
Do not remove amalgams from a pregnant woman.
Further information pertaining to proper amalgam removal can be found on the web page:
http://www.holisticmed.com/dental/amalgam/iaomt.txt
c) Amalgam Removal without Patient Protection
This study measures the mercury level when amalgams are removed not following the protocols presented above.
Molin, M., Bergman B., Marklund, S.L., Schutz, A., Skerfving, S., "Mercury, Selenium, and Glutathione Peroxidase Before and After Amalgam Removal in Man" Acta Odontal Scandinavia; 48:189-202. Oslo. ISSN 0001-6357 (1990).
ABSTRACT: In 10 healthy persons all amalgam fillings were replaced with gold inlays. Blood and urinary levels were measured on 10 occasions during a 4-month period before and a 12-month period after amalgam removal. These variables were also measured three times in 10 healthy controls. A strong statistically significant relation was found between plasma mercury values and both the total number of amalgam surfaces (r=0.71, p=0.0006) and the total surface area of the fillings (r=0.73, p=0.004). In the immediate post removal phase plasma mercury rose by three- to four-fold, whereas the urinary and erythrocyte mercury rose about 50%. These peak values declined to the pre-removal level at about 1 month after removal.
Twelve months after the removal plasma and urinary mercury levels were reduced to 50% and 25%, respectively, of the initial values for the experimental group. Apart from the significantly lower plasma selenium values 5 and 10 days after removal no significant differences were found with regard to plasma selenium or erythrocyte glutathione peroxidase either within or between the experimental and the control groups. A large number of supplementary biochemical analyses did not show any influence on organ functions or any differences between the groups before or after the amalgam removal. Amalgam fillings considerably contributed to the plasma and urinary mercury levels.
d) Amalgam Removal with Patient Protection
This study measures the mercury level when amalgams are removed when not following the IAOMT protocols presented above.
Molin, M., Berglund, J.R., Mackert, J.R., "Kinetics of Mercury in Blood and Urine after Amalgam Removal." J. Dental Research, 74:420,IADR abstract 159, (1995).
ABSTRACT: Even through a number of studies have not been able to reveal any correlation between subjective symptoms and amalgam load, there are still speculations as to whether patients with subjective symptoms related by the patients themselves to their amalgam fillings could have a changed pattern of elimination of mercury. The aim of the present investigation was to study the elimination half-time of mercury in plasma, erythrocytes and urine over an extended period of time after amalgam removal in a group of 10 patients with subjective symptoms by the patients themselves referred to their amalgam fillings and a group of 8 healthy subjects. The average number of occlusal and total amalgam surfaces in the patient group were 13.0 (range 4-20) and 44.4 (range 24-68), respectively. Corresponding figures in the control group were 12.9 (range 10-16) and 40.9 (range 24-63).
The amalgam removal using rubber dam, water spray cutting and high volume vacuum evacuator, was carried out at one and the same time. Blood and urine samples were collected at two occasions before the amalgam removal, then blood was collected at thirty two occasions and urine at forty three occasions during the following year. The mercury content was analyzed by CVAAS technique.
The measured P-, Ery- and U-Hg concentrations before amalgam removal were slightly higher in the control group (6.43.3 nmol/L, 19.46.6 nmol/L, and 2.71.3 nmol/nmol) creatinine respectively than in the symptom group (5.61.8 nmol/L, 14.88.8 nmol/L, and 1.60.9 nmol/nmol) creatinine respectively.
The Hg-concentrations did not significantly increase in the two groups after amalgam removal. Six days after the removal the plasma mean concentration was significantly decreased at P level and ten days after the decrease was at a permanent P level. The mean Ery-Hg level was significantly decreased after eleven days (p), a level that remained stable for the rest of the year. The mean U-Hg level was significantly decreased to one month after the removal and after six months the mean level was reduced with 80 % compared to the initial level in both groups.
The conclusion to be drawn for the present study is that the symptom group did not have a changed pattern of elimination of mercury compared to the healthy group.
Begerow, J., Zander, D., Freier, I., Dunemann, L. "Long-Term Mercury Excretion in Urine After Removal of Amalgam Fillings" International Arch. Occupation Environmental Health 66:209-212 (1994).
ABSTRACT: The long-term urinary mercury excretion was determined in seventeen 28- to 55-year old persons before and at varying times (up to 14 months) after removal of all (4-24) dental amalgam fillings. Before removal the urinary mercury excretion correlated with the number of amalgam fillings. In the immediate post-removal phase (up to 6 days after removal) a mean increase of 30 percent was observed. Within 12 months the geometric mean of the mercury excretion was reduced by a factor of five from 1.44ug/g (range: 0.57 to 4.38ug/g) to 0.35 ug/g (range: 0.13 to 0.88 ug/g).
The exposure from amalgam fillings thus exceeds the exposure from food, air and beverages. Within 12 months after removal of all amalgam fillings the participants showed substantially lower urinary mercury levels which were comparable to those found in subjects who have never had dental amalgam fillings. A relationship between the urinary mercury excretion and adverse effects was not found. Differences in the frequency of effects between the pre- and post-removal phase were not observed.
DISCUSSION: The initial urinary mercury concentrations (before amalgam removal) were similar to those found in previous studies in people with amalgam fillings while the final values (12 months after amalgam removal) were comparable to those for people who have never had amalgam fillings.Our results are in excellent agreement with those of Molin et. al., who found a 75 percent reduction in urinary mercury levels within 12 months after amalgam removal. In accordance with the findings in this study, Molin also found a 50 percent increase in the urinary mercury excretion in the immediate post-removal phase.
Elligsen et. al. and Roels et. al. monitored the urinary mercury excretion after cessation of occupational exposure in a chloralkali plant. The biological half-life was calculated to be 91 days and 90 days, respectively. Both groups of authors concluded that the elimination rate after cessation of mercury exposure seems to be monophasic. This is in agreement with the results of this study based on dental exposure levels.The present study indicates that in persons with amalgam fillings on an average about 80 percent of the urinary mercury excretion is caused by the release from dental amalgam. Thus the inorganic mercury exposure form this source far exceeds the exposure from all other enviornmental sources (food, water, beverages, air).
e) Pregnancy Precaution
The formation of a foetus is very much at risk to mercury in its mother's blood, so the continuous release of mercury from amalgam restorations may be responsible for a portion of the birth defects seen in our society today. When an amalgam filling is removed or an amalgam-filled tooth is extracted, a surge of mercury may be released into the bloodstream. Women should have their amalgam fillings removed at least one year in advance of when they intend to become pregnant and discuss the risk with an informed medical doctor or dentist. Women should never have amalgam fillings removed during a pregnancy.
f) Patient Reports
Siblerud, R.L. "Health Effects After Dental Amalgam Removal" Journal of Orthomolecular Medicine. Vol. 5, No. 2, (1990).
SUMMARY: A Utah dentist provided the names and addresses of approximately 300 people who had their amalgams removed. A health questionnaire was sent to these people; 86 subjects responded. Eighty (80) % of the subjects reported that they felt better following amalgam removal. Nearly all of the subjects (91%) said they were glad their amalgams had been removed and 88% said they would do it again. An increase in happiness and peace of mind was experienced by 58% of the subjects. This evidence suggests that the well being of these subjects improved immensely after amalgam removal.
Mary Davis editor "Solving the Puzzle of Mystery Syndromes" Hot Off the Press Printing Co. 2000
SUMMARY: This book presents patient-reported case histories, where they associate their health problems with dental amalgam mercury. Case histories include: Chronic Fatigue Syndrome, Seizures, Memory Loss, Migraines, Multiple Allergies, Multiple sclerosis, Depression, Lupus, Maldigestion, Chemical Sensitivities, Insomnia, Miscarriages, Paralysis, Sinus Problems, Emotional & Mental Disorders, Infertility, Endometriosis, Crohn's Disease, Rashes, Anxiety, Tremors & Spasms, Amyotrophic Lateral Sclerosis, Universal Reactor and many others.......
MERCURY IS A DEADLY POISON!
 It is surprising how the controversy regarding mercury still continues even though the research indicating that it is a lethal poison is accumulating rapidly.
It is surprising how the controversy regarding mercury still continues even though the research indicating that it is a lethal poison is accumulating rapidly.This research has shown how detrimental mercury can be to the developing foetus, the newborn, the developing child and the adult.
My intention here is to present a few research studies that will show the various detrimental effects of mercury at all stages of life.
Is there a correlation between the number of amalgams and the amount of mercury excreted in the urine after provocation?
ABSTRACT:
There is a considerable controversy as to whether dental amalgams may cause systemic health effects in humans because they liberate elemental mercury. Most such amalgams contain as much as 50% metallic mercury.
To determine the influence of dental amalgams on the mercury body burden of humans, we have given volunteers, with and without amalgams in their mouth, the sodium salt of 2, 3-dimercaptopropane-1-sulfonic acid (DMPS), a chelating agent safely used in the Soviet Union and West Germany for a number of years. The diameters of dental amalgams of the subjects were determined to obtain the amalgam score.
Administration of 300 mg DMPS by mouth increased the mean urinary mercury excretion of the amalgam group from 0.70 to 17.2 ug and that of the non amalgam group from 0.27 to 5.1 ug over a 9 hour period.
Two-thirds of the mercury excreted in the urine of those with dental amalgams appears to be derived originally from the mercury vapor released from their amalgams.
Linear regression analysis indicated a highly significant positive correlation between the mercury excreted in the urine 2 hours after DMPS administration and the dental amalgam scores. DMPS can be used to increase the urinary excretion of mercury and thus increase the significance and reliability of this measure of mercury exposure or burden, especially in cases of micromercurialism.
Aposhian, H.V., D.C. Bruce, W. Alter, R.C. Dart, K.M. Hurlbut, M.M. Aposhian, "Urinary Mercury after Administration of 2, 3-dimercaptopropane-1-sulfonic acid: Correlation with Dental Amalgam Score" FASEB J. 6: 2472-2476; (1992).
Can dental mercury release from the mother be detected in the foetus?
ABSTRACT:
In humans, the continuous release of Hg vapour from dental amalgam tooth restorations is markedly increased for prolonged periods after chewing. The present study establishes a time-course distribution for amalgam, Hg in body tissues of adult and foetal sheep. Under general anaesthesia, five pregnant ewes had twelve occlusal amalgam fillings containing radioactive 203Hg placed in teeth at 112 days gestation.
Blood, amniotic fluid, faeces, and urine specimens were collected at 1- to 3-day intervals for 16 days. From days 16-140 after amalgam placement (16-41 days for foetal lambs), tissue specimens were analyzed for radioactivity, and total Hg concentrations were calculated. Results demonstrate that Hg from dental amalgam will appear in maternal and foetal blood and amniotic fluid within 2 days after placement of amalgam tooth restorations.
Excretion of some of this Hg will also commence within 2 days. All tissues examined displayed Hg accumulation. Highest concentrations of Hg from amalgam in the adult occurred in kidney and liver, whereas in the foetus the highest amalgam Hg concentrations appeared in the liver and pituitary glands. The placenta progressively concentrated Hg as gestation advanced to term, and milk concentration of amalgam Hg postpartum provides a potential source of Hg exposure to the newborn. It is concluded that accumulation of amalgam Hg progresses in maternal and foetal tissues to a steady state with advancing gestation and is maintained.Vimy, M.J., Y. Takahashi, and F.L. Lorscheider "Maternal-foetal distribution of mercury (203Hg) released from dental amalgam fillings." Am. J. Physiol. 258 (Regulatory Integrative Comp. Physiol. 27): R939-R945 (1990).
Can in utero exposure to mercury cause behavioural disturbances?
ABSTRACT:
Pregnant rats were either 1) administered methyl mercury (MeHg) by gavage, 2 mg/kg/day during days 6-9 of gestation, 2) exposed by inhalation to metallic mercury (Hg) vapour (1.8 mg/m3 air for 1.5 hours per day) during gestation days 14-19, 3) exposed to both MeHg by gavage and Hg vapour by inhalation (MeHg + Hg), or 4) were given combined vehicle administration for each of the two treatments (control).
The inhalation regimen corresponded to an approximate dose of 0.1 mg Hg/kg/day.Clinical observations and developmental markers up to weaning showed no differences between any of the groups. Testing of behavioural functions was performed between 4 and 5 months of age and included spontaneous motor activity, spatial learning in a circular path, and instrumental maze learning for food reward.
Offspring of dams exposed to Hg vapour showed hyperactivity in the motor activity test chambers over all three parameters: locomotion, rearing and total activity; this effect was potentiated in the animals of the MeHg + Hg group. In the swim maze test, the MeHg + Hg and Hg groups evidenced longer latencies to reach a submerged platform, which they had learned to mount the day before, compared to either the control or MeHg group.
In the modified, enclosed radial arm maze, both the MeHg + Hg and Hg groups showed more ambulations and rearings in the activity test prior to the learning test. During the learning trial, the same groups (i.e., MeHg + Hg and Hg) showed longer latencies and made more errors in acquiring all eight pellets.Fredriksson, A., Dencker, L., Archer, T., Danielsson, B.R. "Prenatal Coexposure to Metallic Mercury Vapor and Methyl Mercury Produce Interactive Behavioral Changes in Adult Rats." Neurotoxicol Teratol., 18(2): 129-34, (1996).
ABSTRACT: The total mercury concentrations in the liver (Hg-L), the kidney cortex (Hg-K) and the cerebral cortex (Hg-C) of 108 children aged 1 day- 5 years, and the Hg-K and Hg-L of 46 foetuses were determined. As far as possible, the mothers were interviewed and their dental status was recorded.
The results were compared to mercury concentrations in the tissues of adults for the same geographical area. The Hg-K (n=38) and Hg-L (n=40) of foetuses and Hg-K (n=35) and Hg-C (n=35) of older infants (11-50 weeks of life) correlated significantly with the number of dental amalgam fillings of the mother. The toxicological relevance of the unexpected high Hg-K of older infants from mother with higher numbers of dental amalgam fillings is discussed. Conclusion: Future discussion on the pros and cons of dental amalgam should not be limited to adults or children with their own amalgam fillings, but also include foetal exposure.
The unrestricted application of amalgam for dental restorations in women before and during the child-bearing age should be reconsidered. Abbreviations: Hg-C total mercury concentration in the cerebral cortex (ng/g wet weight). Hg-K total mercury concentration in the renal cortex (ng/g wet weight). Hg-L total mercury concentration in the liver (ng/g wet weight).Drasch et. al. "Mercury Burden of Human Fetal and Infant Tissues" European Journal of Pediatrics (August 1994).
Can mercury amalgam from lactating mothers affect the foetus in utero?
ABSTRACT: Neonatal uptake of Hg from milk was examined in a pregnant sheep model, where radioactive mercury (Hg203)/silver tooth fillings (amalgam) were newly placed. A crossover experimental design was used in which lactating ewes nursed foster lambs. In a parallel study, the relationship between dental history and breast milk concentration of Hg was also examined.Results from the animal studies showed that, during pregnancy, a primary fetal site of amalgam, Hg concentration is in the liver, and after delivery the neonatal lamb kidney receives additional amalgam Hg from mother's milk.
In lactating women with aged amalgam fillings, increased Hg excretion in breast milk and urine correlated with the number of fillings or Hg vapor concentration levels in mouth air.It was concluded that Hg originating from maternal amalgam tooth fillings transfers across the placenta to the fetus, across the mammary gland into milk ingested by the newborn and ultimately into neonatal body tissues.
Comparisons are made to the U.S. minimal risk level recently established for adult Hg exposure. These findings suggest the placement and removal of "silver" tooth filings in pregnant and lactating humans will subject the fetus and neonate to unnecessary risk of Hg exposure.Vimy, M.J., Hooper, D.E., King, W.W., Lorscheider, F.L., "Mercury from Maternal "Silver" Tooth Fillings in Sheep and Human Breast Milk: A Source of Neonatal Exposure" Biological Trace Element Research, 56:143-52, (1997).
Can heavy metals affect human fertility?
ABSTRACT: Heavy metals have been identified as factors affecting human fertility. This study was designed to investigate whether the urinary heavy metal excretion is associated with different factors of infertility.
The urinary heavy metal excretion was determined in 501 infertile women after oral administration of the chelating agent 2,3-dimercaptopropane-1-sulfonic acid (DMPS). Furthermore, the influence of trace element and vitamin administration on metal excretion was investigated. Significant correlations were found between different heavy metals and clinical parameters (age, body mass index, nationality) as well as gynaecological conditions (uterine fibroids, miscarriages, hormonal disorders).
Diagnosis and reduction of an increased heavy metal body load improved the spontaneous conception chances of infertile women. The DMPS test was a useful and complementary diagnostic method. Adequate treatment provides successful alternatives to conventional hormonal therapy.Gerhard, I., Monga, B., Waldbrenner, A., Runnebaum, B., "Heavy Metals and Fertility" Journal of Toxicology and Environmental Health, Part, A, 54:593-611, (1998).
Is mercury associated with cardiac dysfunction?
OBJECTIVES: We sought to investigate the possible pathogenetic role of myocardial trace elements (TE) in patients with various forms of cardiac failure.BACKGROUND: Both myocardial TE accumulation and deficiency have been associated with the development of heart failure indistinguishable from an idiopathic dilated cardiomyopathy.
METHODS: Myocardial and muscular content of 32 TE has been assessed in biopsy samples of 13 patients (pts) with clinical, hemodynamic and histologic diagnosis of idiopathic dilated cardiomyopathy (IDCM), all without past or current exposure to TE.
One muscular and one left ventricular (LV) endomyocardial specimen from each patient, drawn with metal contamination-free technique, were analyzed by neutron activation analysis and compared with
1) similar surgical samples from patients with valvular (12 pts)and ischemic (13 pts) heart disease comparable for age and degree of LV dysfunction;
2) papillary and skeletal muscle surgical biopsies from 10 pts with mitral stenosis and normal LV function, and
3) LV endomyocardial biopsies from four normal subjects.
RESULTS: A large increase (>10,000 times for mercury and antimony) of TE concentration has been observed in myocardial but not in muscular samples in all pts with IDCM.
Patients with secondary cardiac dysfunction had mild increase (< or = 5 times) of myocardial TE and normal muscular TE. In particular, in pts with IDCM mean mercury concentration was 22,000 times (178,400 ng/g vs. 8 ng/g), antimony 12,000 times (19,260 ng/g vs. 1.5 ng/g), gold 11 times (26 ng/g vs. 2.3 ng/g), chromium 13 times (2,300 ng/g vs. 177 ng/g) and cobalt 4 times (86,5 ng/g vs. 20 ng/g) higher than in control subjects.
CONCLUSIONS: A large, significant increase of myocardial TE is present in IDCM but not in secondary cardiac dysfunction. The increased concentration of TE in pts with IDCM may adversely affect mitochondrial activity and myocardial metabolism and worsen cellular function.Frustaci A, Magnavita N, Chimenti C, Caldarulo M, Sabbioni E, Pietra R, Cellini C, Possati GF, Maseri A. Department of Cardiology, Catholic University, Rome, Italy. "Marked elevation of myocardial trace elements in idiopathic dilated cardiomyopathy compared with secondary cardiac dysfunction." From: J Am Coll Cardiol 1999 May;33(6):1578-83
Can dental mercury provoke an increase in antibiotic-resistant bacteria in oral and intestinal flora?
ABSTRACT: In a survey of 640 human subjects, a subgroup of 356 persons without recent exposure to antibiotics demonstrated that those with a high prevalence of Hg resistance in their intestinal floras were significantly more likely to also have resistance to two or more antibiotics. This observation led us to consider the possibility that mercury released from amalgam ("silver") dental restorations might be a selective agent for both mercury- and antibiotic-resistant bacteria in the oral and intestinal floras of primates.
Resistances to mercury and the several antibiotics were examined in the oral and intestinal floras of six adult monkeys prior the installation of amalgam fillings, during the time they were in place, and after replacement of the amalgam fillings with glass ionomer fillings (in four of the monkeys). The monkeys were fed an antibiotic-free diet, and fecal mercury concentrations were monitored.
There was a statistically significant increase in the incidence of mercury-resistant bacteria during the 5 weeks following installation of the amalgam fillings and during the 5 weeks immediately following their replacement with glass ionomer fillings. These peaks in incidence of mercury-resistant bacteria correlated with peaks of Hg elimination (as high as 1mM in the faeces) immediately following amalgam placement and immediately after replacement of the amalgam fillings.
Representative mercury-resistant isolates of three selected bacterial families (oral streptococci, members of the family Enterobacteriaceae, and enterocaocci) were also resistant to one or more antibiotics, including ampicillin, tetracycline, streptomycin, kanamycin, and chloramphenicol. While such mercury- and antibiotic-resistant isolates among the staphylococci, the enterococci, and members of the family Enterobacteriaceae, have been described, this is the first report of mercury resistance in the oral streptococci.
Many of the enterobacterial strains were able to transfer mercury and antibiotic resistances together to laboratory bacterial recipients, suggesting that the loci for these resistances are genetically linked.
Our findings indicate that mercury released from amalgam fillings can cause an enrichment of mercury resistance plasmids in the normal bacterial floras of primates. Many of these plasmids also carry antibiotic resistance, implicating the exposure to mercury from dental amalgams in an increased incidence of multiple antibiotic resistance plasmids in the normal floras of nonmedicated subjects.
Summers, A.O., J.Wireman, M.J. Vimy, F.L. Lorscheider, B. Marshall, S.B. Levy, S. Bennett, and L. Billard, "Mercury Released form Dental "Silver" Fillings Provokes an Increase in Mercury- and Antibiotic-Resistant Bacteria in Oral and Intestinal Floras of Primates", Antimicrobial Agents and Chemotherapy, (April 1993), pages 825 - 834.
Are there increased blood mercury levels in patients with Alzheimer's Disease?
SUMMARY: Alzheimer's disease (AD) is a common neurodegenerative disorder that leads to dementia and death. In addition to several genetic parameters, various environmental factors may influence the risk of getting AD.
In order to test whether blood levels of the heavy metal mercury are increased in AD, we measured blood mercury concentrations in AD patients (n=33), and compared them to age-matched control patients with major depression (MD) (n=45), as well as to an additional control group of patients with various non psychiatric disorders (n=65). Blood mercury levels were more than two fold higher in AD patients as compared to both control groups (p=0.0005, and p=0.0000, respectively). In early onset AD patients (n=13), blood mercury levels were almost three fold higher as compared to controls (p=0.0002, and p=0.0000, respectively).
These increases were unrelated to the patients' dental status. Linear regression analysis of blood mercury concentrations and CSF levels of amyloid B-peptide (AB) revealed a significant correlation of these measures in AD patients (n=15, r=0.7440, p=0.0015, Pearson type of correlation).
These results demonstrate elevated blood levels of mercury in AD, and they suggest that this increase of mercury levels is associated with high CSF levels of AB, whereas tau levels were unrelated. Possible explanations of increased blood mercury levels in AD include yet unidentified environmental sources or release from brain tissue with the advance in neuronal death.C. Hock, G. Drasch, S. Golombowski, F. Muller-Spahn, B. Willershausen-Zonnchen, P. Schwarz, U. Hock, J.H. Growdon, R.M. Nitsch "Increased Blood Mercury Levels in Patients with Alzheimer's Disease" Journal of Neural Transmission, 105: (1998).
Chemical & Heavy Metal Cleanse Starter Kit$149.85  The Chemical & Heavy Metal Starter Kit was designed by Dr. Group for individuals that are new to the cleansing process, or are simply looking for an easy-to-perform, cost effective cleanse program. The Heavy Metal Starter Kit is comprised of LIFE Detox Foot Patches™, NDF Plus™, and Quantum Zeolite™. |
Tuesday, November 07, 2006
Mercury Poisoning
 The Calcium EDTA in Detoxamin has an extra chemical bond compared to the older Disodium EDTA. This gives Detoxamin EDTA an affinity for Mercury.
The Calcium EDTA in Detoxamin has an extra chemical bond compared to the older Disodium EDTA. This gives Detoxamin EDTA an affinity for Mercury.Mercury is also excreted from the body through the feces, because Detoxamin utilizes the colon wall for EDTA assimilation, it is a powerful Mercury chelator, making it possible to eliminate DMPS in some patients.
Detoxamin has a time-release mechanism that allows the EDTA to absorb through the colon wall over an eighty-minute period while you sleep, through a time-release mechanism.
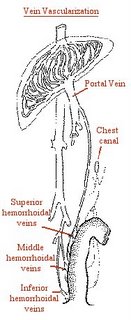
Almost all the blood from the rectum makes its way to the superior hemorrhoidal veins, a tributary of the portal system, so that absorption through the rectal wall carries the EDTA in Detoxamin to the portal vein (see figure).
The lower and middle hemorrhoidal veins bypass the liver-and do not undergo first pass metabolism. This means that the EDTA in Detoxamin goes directly to the organs of your body without being filtered through the liver first. Because of this, the EDTA contained in Detoxamin is very productive.
Detoxamin offers many advantages both over the expensive intravenous method of EDTA chelation. With the use of needles via the intravenous method, and risk of AIDS and other communicable blood-borne diseases, Detoxamin is becoming the logical choice over I.V. EDTA chelation and the poorly absorbed oral EDTA.
The rectum has a more neutral pH and is not as acidic as the stomach, which makes this area much better for EDTA absorption because it is not buffered and has a neutral pH, unlike the stomach. It also has very little enzymatic activity, thus enzymatic degradation does not occur.
The rectal mucosa (rectum) is much more capable than the gastric mucosa (stomach) of tolerating various drug-related irritations. This is why patients who can't tolerate oral pain medication are given the same medication in suppository form. In fact, absorption with any oral EDTA tablet is so low that 135 (500mg) oral EDTA tablets are equal to just 5 Detoxamin suppositories.
Detoxamin also introduces EDTA directly into the systemic circulation, efficiently bypassing the portal circulation and the liver metabolism on the first pass. Rectal absorption may also occur through the lymphatic system and, in some cases, largely through the blood via the vena cava.
An often overlooked, but extremely important source of toxic material is the mercury from silver [mercury] amalgam fillings. Some people who are aware of the situation are confused by the mixture of information available. Unfortunately, statements from dental trade organizations and on a few poorly-researched news reports have muddled the situation.Here are a few facts about mercury amalgam fillings:
Causes Damage to Brain in ChildrenIn February, 1998, a group of the world's top mercury researchers announced that mercury from amalgam fillings can permanently damage the brain, kidneys, and immune system of children.
Amalgam Fillings Linked to Neurological Problems, Gastrointestinal ProblemsThe first large-scale epidemiological study of mercury and adverse reactions was recently completed and showed that of the symptoms looked at, there was a link seen to gastrointestinal problems, sleep disturbances, concentration problems, memory disturbances, lack of initiative, restlessness, bleeding gums and other mouth disorders.
Mercury / Alzheimer's Disease Connection Found
A study related to mercury and Alzheimer's Disease was recently completed by a team of scientists led by well-respected researcher Dr. Boyd Haley. They exposed rats to levels of mercury vapor diluted to account for size differences between humans and rats. The rats developed tissue damage "indistinguishable" from that of Alzheimer's Disease. Repeating the experiment showed the same results. Dr. Haley is quoted as saying "I'm getting the rest of my fillings taken out right now, and I've asked my wife to have hers replaced too." Also see: http://www.holistic-dentistry.com/artalzeimer.asp
Amalgam Fillings Since 1970s Unstable
The type of mercury fillings that began to be used during the last couple of decades, non-gamma-2 (high copper), releases many times more mercury than the older style of amalgam fillings. Also, please see the article on the instability of dental amalgam fillings by Ulf Bengsston.
Amalgam Fillings Release Highly Toxic Elemental MercuryMercury is one of the most toxic substances known. The mercury release from fillings is absorbed primarily as highly toxic elemental mercury vapor.
Amalgam Fillings Largest Source of Mercury By FarBased on a number of studies in Sweden, the World Health Organization review of inorganic mercury in 1991 determined that mercury absorption is estimated to be approximately four times higher from amalgam fillings than from fish consumption. Recent studies have confirmed this estimate. The amount absorbed can vary considerably from person to person.
Gold Crowns, Gum, Bruxism, Computer Monitors Increase Release of Mercury Significantly
Gum chewing, grinding of teeth/bruxism, computer terminal exposure, presence of gold fillings or gold crowns (even if covering mercury fillings), teeth brushing, braces, and chewing cause the release of significantly increased amounts of mercury from the fillings.
Also, please see the following short review related to increases in mercury exposure from dissimilar metals in the mouth, exposure to magnetic fields, chewing, etc.
Cumulative Poison and Builds Up in Organs
Mercury released from fillings builds up in the brain, pituitary, adrenals, and other parts of the body.
Mercury Amalgam Fillings Effect PorphyrinsPreliminary results from the first detailed biochemical analysis (scroll half-way down) of patients who removed mercury amalgam fillings showed a significant drop in the excretion of porphyrins (important to heme synthesis -- heme carries oxygen to red blood cells), as well as a number of other key biochemical changes. Also, see the Video of the preliminary results from the study.
Potential Contribuatory Factor in Other DiseasesMercury from amalgam fillings has been implicated as a possible contribuatory factor in some cases of Multiple sclerosis, Parkinson's Disease, IBS, reproductive disorders, allergies, and a variety of other illnesses.
Mercury Build Up in Brain, Organs and Breast Milk of Fetuses of Mothers With Amalgam FillingsMercury from fillings in pregnant women has been shown to cause mercury accumulation in brain, kidneys and liver of human fetuses (all of the areas tested). Studies have shown that mercury can be passed to infants from breast milk.
Proper Removal of Fillings Produces Eventual Health ImprovementA recent study published in the Journal of Orthomolecular Medicine related to the proper removal of mercury amalgam fillings from 118 subjects showed an elimination or reduction or 80% of the classic mercury poisoning symptoms. In many cases, it took 6 to 12 months after mercury amalgam removal for the symptoms to disappear.
World-reknowned Experts Agree About Potential DangerIn contrast to statements from dental trade organizations, toxicologists and medical researchers are often quite concerned about the use of mercury. Lars Friberg, the lead toxicologist on the World Health Organization team looking at inorganic mercury and health effects recently stated that he believes that mercury is unsuitable for dental materials because of safety concerns.
Canadian Class Action LawsuitCanadians are in the process of beginning a major class action lawsuit based on the fact that the government knew of but did not warn the public about mercury dangers from amalgam fillings.
Legal actions related to mercury exposure from mercury amalgam fillings and vaccines are beginning in the United States. For more information and a directory of Mercury-free dentists, please see the TalkInternation.com web site.Obviously, not everyone experiences acute toxicity effects from the mercury in amalgam fillings. However, virtually everyone does have mercury build up in their bodies from implantation of such fillings.
The large increase in mercury exposure from the newer non-gamma-2 mercury fillings means that only time will tell how much damage has been caused by daily exposure to mercury to such fillings.I do not recommend that people assume automatically that they will be healed by the removal of amalgam fillings.
Many people are helped tremendously and some are healed. The 80% figure for people showing improvement within a year likely refers to people who had good reason to suspect that they were being significantly effected by the fillings.
The percentage of people in the general population who might experience health improvement within one year after removal is probably much lower than 80%.
I recommend going into the mercury amalgam removal procedure knowing that, at the very least, you will have removed yourself from a regular exposure to an extremely toxic material such that it will not build up in your organs and possibly cause significant health problems at a later date.
Mercury amalgam fillings should be removed only by dentists with experience using the IOAMT mercury amalgam removal protocol (presented with the permission of the excellent Preventive Dental Association web page).
Such dentists are often experienced with proper evaluation and placement of composite fillings, both of which can be crucial for the success of the treatment.
Biocompatability tests are often important in determining which composite materials can be safely used. I believe that composite (plastic) fillings are a better replacement than metal (e.g., gold) fillings even in chemically-sensitive individuals.
They are, however, not without safety questions, but are still likely to be much less toxic than mercury amalgam fillings. Proper placement of composites should be left to experienced amalgam removal dentists as the average well-meaning dentist may not be aware of the newer placement techniques.
Further scientific information can be found at Mercury Adverse Effects Web Page, 150 Year's of Russian Roulette Web Page, Alt Corp's Amalgam Page, and Bo Walhjalt's Mercury Articles Web Page.
More information about removal, detoxification, and placement of composite fillings can be found at Bioprobe, Inc. and at the Preventive Dental Association.
Information about finding a dentist practicing non-toxic dentistry can be found on the Resources For Related to Non-Toxic Dentistry web page.
Also, the AMALGAM mailing list can be a good source of accurate, up-to-date information.
Important Links
Alt Corp Amalgam Web Page (Scientific links on left of page)
Adverse Reaction Samples From the Internet - Updated 7/01/96
Amalgam-Free Dentists (T.E.S.T.)
Amalgam-Free Dentists (Talk International)
Bikerchick Amalgam Poisoning web page
Bio-Probe, Inc. -- Scientific/General/Detox Book & Product Catalog
Blazing Tattles 3-Part Series on Cleaning Up Dental Work
Canadians For Mercury Relief (CFMR)
Chronic Illness and Chronic Mercury Exposure -- Dr. Edelson's Page
Clifford Consulting & Research (Dental Materials Compatibility Testing)
Consumers for Dental Choice
Dental amalgam - 150 years of Russian Roulette (Dagfinn Reiers¿l)
Hal A. Huggins, DDS, MS - Research In Toxicity
Mercury/Amalgam Fillings-Related Illness FAQ
Vimy Dental Group Web page
Preventive Dental Health Association
more discussion: Forum
· Addiction Forum · Ask the Doctors Forum · Ayurveda Forum · Ayurvedic & Thai Herbs Forum · Colon Cleansing Forum · Dental Forum · Diabetes Forum · Diet Forum · General Cleansing Forum · Hepatitis A, B. C Forum · Integrated Medicine Forum · Live Blood Analysis Forum · Ozone-Oxygen-Forum · pH - Alkaline - Acidity Forum · Weight Loss Forum
Tuesday, July 04, 2006
Protocol for Amalgam-Mercury-Silver Filling Removal
 Protocol for Amalgam-Mercury-Silver Filling Removal ByInternational Academy of Oral Medicine and Toxicology
Protocol for Amalgam-Mercury-Silver Filling Removal ByInternational Academy of Oral Medicine and ToxicologySTAFF PROTECTION
OSHA4 5 requires that employees be given written informed consent before the use of any toxic chemicals of which mercury is one. Elemental mercury vapor is one of the most toxic forms of mercury and should not breathed. Women of child bearing age should be exposed to no more than 10% of the OSHA MAC6. Women who are pregnant should be exposed to no mercury.7 If you use mercury or remove mercury in any form the National Institute of Occupational Safety and Health (NIOSH) has recommended that your employees be medically monitored annually.
ANY MERCURY EXPOSURE REQUIRES THAT THE EMPLOYEE WEAR AN APPROVED MERCURY FILTER MASK.An approved mask is appropriate for wearing during all dental procedures which will expose you or your staff to mercury.8 The manner in which dentists operate their equipment dramatically affects the amount of mercury released. Never drill on mercury high dry. It is hazardous to you, your staff, and your patient. Levels as high as 4000 m g/M3 have been measured 18" from the drill when used high dry. Levels over 1000 m g/M3 are measurable upon opening an amalgam mixing capsule.
One out of 7 California dental offices tested over the OSHA TWA of 50 m g/M 3 . 100% of the vacuum cleaner exhaust tested over 100 m g/M 3 . Any office where mercury is used should be tested regularly and staff should be monitored for exposure. Testing services are available and a mercury sensor badge is available for personnel monitoring. They should test inside storage areas and along baseboards where mercury might have dropped. Office spills can go undetected for years and are extremely hazardous.
Visit our: Dental Forum
Monday, June 26, 2006
Dental Mercury Detox
Sam Ziff, Michael F. Ziff, and Mats Hanson, Ph.D.
Revised and updated 2001 Edition now contains 104 pages and outlines the various nutritional supplements and drugs reflected in the scientific literature that have the potential to reduce mercury body burdens. A new Appendix has been added about the arsenic and lead in fluoride. This is a well researched book written in an easy to read and understand style. If you are contemplating having your mercury/amalgam dental fillings replaced, this is a must read book before you start the process. It covers pre-removal protocols, post-removal protocols, and covers a wealth of other information important to your health and well-being. The new Appendix on fluoride explains that over 90% of the fluoride used to fluoridate drinking water is an industrial waste product that can contian excessive levels of arsenic and lead.
Monday, June 05, 2006
BIOLOGIC DENTISTRY
 BIOLOGIC DENTISTRYby Javier Morales P., D.D.S.
BIOLOGIC DENTISTRYby Javier Morales P., D.D.S."The blood that runs through your tooth will run through your toe in one minute."-Timothy A. Kersten, D.D.S.
AFTER MERCURY AND/OR METHYL MERCURY ENTERS THE BODY, WHAT DAMAGE CAN IT DO?
1. Combine with enzymes and proteins and inactivate them for normal body function.
2. ...[it can] combine with a transport system like hemoglobin so it cannot act it's normal function....[With hemoglobin], the result would be decreased oxygen transport.
3. It can be combined with other metals...creating a battery in the mouth capable of generating electric current.
4. Mercury very easily enters our body's cells and can destroy the DNA or nuclear material within those cells. This can lead to premature aging or reduce[d] resistance. Normal cells live for a short span, then duplicate themselves. Mercury injured cells die without replacing themselves.
THERE ARE 5 BASIC AREAS WHERE AMALGAM REMOVAL HAS BEEN ASSOCIATED WITH IMPROVEMENTS IN HEALTH....
1. NEUROLOGICAL.... a) Emotional (especially depression, irritability, suicidal tendancies, inability to cope). b) Motor (such as multiple sclerosis, seizures, facial twitches, muscle spasms).
2. CARDIOVASCULAR (Endocarditis, heart valves, or lungs).
3. COLLAGEN DISEASES (Problems with the cementing sub- stances of the cells such as scleroderma, arthritis, lupus, bursitis).
4. IMMUNOLOGICAL....Interference...[caused by mercury] gives you more suseptibility to catch whatever diseases are 'going around'.
5. ALLERGIES (Foods, airborn, universal reactors. Mercury in combination with what you are 'allergic to' will rupture white blood cells and can precipitate...an allergic reaction....
Fillings have two types of electrical current in them, electro- positive and electro-negative. ...When positively charged fillings are removed while leaving negative ones behind, the patient often feels worse.... [And], their chances for improvement drop[s] drastically. If negatively charged fillings are removed first, there is not much 'withdrawl' feeling and chances for success ...approach the 80% mark.
With scientific technology now on the cutting edge, research on Bioelectricity and its effects on the body is just now being... [observed].
Four states have passed legislation that either ban[s] the use of mercury altogether or...[requires]...dentist[s]...provide the patient with information regarding the possible side effects.... Interesting!!
Dental Forum at:
http://dreddyclinic.com/forum/viewforum.php?f=5
Wednesday, January 04, 2006
Mercury and ALS
 This is from the ALS web site
This is from the ALS web siteEffects of Mercury Toxicity
Several scientific reports have suggested a possible relationship between certain chronic or unexplained illnesses and the presence of mercury in the body.It appears that toxic substances, such as mercury, may accumulate in the body and have the potential to damage the brain, heart, lungs, liver, kidney, bloodcells, hormones and suppress the body's immune system.
Some researchers are looking at the possibility that mercury toxicity may play a role in the development of multiple sclerosis, Parkinson's and Alzheimer's diseases.
A human autopsy study comparing the brain tissue of people with Alzheimer's disease, with an aged matched group of brains from people without Alzheimer's Disease, showed the Alzheimer's group to have a significant higher concentration of mercury in all the areas of the brains involved in memory function. Mercury poisoning in people exposed to large amounts of industrial or environmental mercury has been studied. Its relationship to severe symptoms and illness has been well documented.
High levels of mercury have been linked to a weakened immune system and candidiasis.
Improvement has occurred in some individuals after the removal of mercury and mercury detoxification. Improvements have been reported in kidney function, reversal of some mental and neurologic symptoms, thought and memory disturbances, chronic fatigue and the reversal of other symptoms as mentioned above, which have been thought to be due to chronic and untreatable illness.The sources of mercury contamination have been very controversial. However, a growing number of research scientists feel that a major source is the mercury found in dental amalgams, with lesser amounts from fish, seafood, other foods, contaminated water and air pollutants.
For more than 150 years, dentistry has used silver mercury Amalgam (commonly referred to as "silver" fillings), which contains approximately 50% mercury metal as the preferred tooth filling material. Medical research has demonstrated that this mercury can be released as a vapor into the mouth. It is then inhaled and absorbed into the body tissues where it eventually becomes tightly bound to cell protein. A review of studies on mercury toxicity and dental amalgam, by Lorscheider, Vimy and Summers, concludes that the animal and human experiments to date, demonstrate that the uptake, tissue distribution and excretion of amalgam mercury is significant and that dental amalgam is the major contributing source to the mercury body burden in humans.
It has been felt that mercury in dental amalgam is safely bound. However, growing evidence again suggests that mercury can enter the body through the vapors. Chewing, grinding the teeth, hot food or drink and even the corrosive action by our saliva may cause these vapors to be released. Sheep exposed to mercury dental fillings, after 30 days of chewing, showed significant loss of kidney function.
Integrated Medicine Dental Forum
http://www.DrEddyClinic.com/
http://dreddyclinic.com/forum/viewforum.php?f=4
Mercury-Caused Endocrine Conditions Causing Widespread Adverse Health Effects,Cognitive Effects, and Fertility Effects B.Windham(Ed.)
 Mercury-Caused Endocrine Conditions Causing Widespread Adverse Health Effects, Cognitive Effects, and Fertility Effects B.Windham(Ed.)http://www.home.earthlink.net/~berniew1/endohg.html
Mercury-Caused Endocrine Conditions Causing Widespread Adverse Health Effects, Cognitive Effects, and Fertility Effects B.Windham(Ed.)http://www.home.earthlink.net/~berniew1/endohg.htmlIntroduction.
As will be documented in this paper, the majority of the population receives significant mercury exposures and significant adverse health effects are common.
Mercury has been found to be an endocrine system disrupting chemical in animals and people, disrupting function of the pituitary gland, thyroid gland, thymus gland, adrenal gland, enzyme production processes, and affecting many hormonal functions at very low levels of exposure.
The main factors determining whether chronic conditions are induced by metals appear to be exposure and genetic susceptibility, which determines individuals immune sensitivity and ability to detoxify metals(405). Very low levels of exposure have been found to seriously affect large groups of individuals who are immune sensitive to toxic metals, or have an inability to detoxify metals due to such as deficient sulfoxidation or metallothionein function or other inhibited enzymatic processes related to detoxification or excretion of metals.
Common Exposures to Significant Levels of Mercury and Distribution in the Body
Most people with several Amalgam fillings get daily exposure of mercury at levels well above
U.S. government health guidelines(16,19,20,49,199,211,500 ), which amount to about 4 to 8 micrograms per day(217). Mixed metals in the mouth such as amalgam dental fillings, metal crowns, and metal braces have been found to result in galvanic currents in the metals which drive the metals into the saliva and tissues of the oral cavity at high levels as well as systemically, with accumulations in the brain and hormonal glands(84,85,192,348,369,381,500).
Additionally, electric and electromagnetic fields from appliances, computer monitors, power lines, etc. cause electric currents in metals in the mouth which further increase exposures to mercury and other metals(28). Mercury and nickel, which are highly neurotoxic (19,84,217,372,453,500) and immunotoxic(181,91,114ab,380b,369,383ab,405), are often found at high levels in tests of those with mixed metals in the mouth and are known to commonly cause DNA damage(296,458,114), immune reactivity (234,330,331,342,369,375,383,405,91), and hormonal effects in animals and humans(50,84,104,105,369,382,459), including related reproductive effects. Government health agencies in other countries such as Health Canada and amalgam manufacturers have warned against using amalgam near other metals(287,500), but this is still common in the U.S. and several other countries.
Children typically also get high levels of exposure to highly toxic organic mercury compounds such as ethyl mercury through thimerosal, used as a preservative in vaccines (160,409,476,555), and to methyl mercury from fish(2). Warnings to ban or limit consumption of fish have been issued for 20 percent of all U.S. lakes, including all Great Lakes, as well as 7 percent of all U.S. river miles and many bays(2).
Mercury (especially mercury vapor or organic mercury) rapidly crosses the blood brain barrier and is stored preferentially in the pituitary gland, thyroid gland, hypothalamus, and occipital cortex in direct proportion to the number and extent of dental amalgam surfaces (14,16,19,85,99,211,273,274,287,327,348,366,369,453), and likewise rapidly crosses the placenta and accumulates in the fetal brain and hormonal glands at levels commonly higher than the level in the mother(20,28,50,61,500). Thus mercury has a greater effect on the functions of these areas. Mercury exposures from the various sources have been found to be cumulative and synergistic along with exposures to other toxic metals(500).
Endocrine System Effects of Mercury and Related Neurological and Immune Effects
Studies have documented that mercury causes hypothyroidism(50,390,35), damage of thyroid RNA(458), autoimmune thyroiditis(369,382,91), and impairment of conversion of thyroid T4 hormone to the active T3 form(369,382,390,459,35,50d). In general immune activation from toxics such as heavy metals resulting in cytokine release and abnormalities of the hypothalamus-pituitary-adrenal axis can cause changes in the brain, fatigue, and severe psychological symptoms(369,375,379-382, 385,453,118) such as profound fatigue, muscoskeletal pain, sleep disturbances, gastrointestinal and neurological problems as are seen in CFS, Fibromyalgia, and autoimmune thyroiditis..
Such hypersensitivity has been found most common in those with genetic predisposition to heavy metal sensitivity (342,369,382,405), such as found more frequently in patients with HLA-DRA antigens (375,381,383). A significant portion of the population appears to fall in this category. Mercury is documented to accumulate in the gastrointestinal tract(19,20) and commonly causes "leaky gut" and poor absorption which are a factor in these conditions(21,338). Such symptoms usually improve significantly after amalgam removal(500).
Mercury can have significant effects on thyroid function even though the main hormone levels remain in the normal range, so the usual thyroid tests are not adequate in such cases. Prenatal methylmercury exposure severely affects the activity of selenoenzymes, including glutathione peroxidase (GPx) and 5-iodothyronine deiodinases(5-Di and 5'-DI) in the fetal brain, even though thyroxine(T4) levels are normal(390e). Gpx activity is severely inhibited, while 5-DI levels are decreased and 5'-DI increased in the fetal brain, similar to hypothyroidism. Thus normal thyroid tests will not pick up this condition.
Mercury reduces the bloods ability to transport oxygen to fetus and transport of essential nutrients including amino acids, glucose, magnesium, zinc and Vit B12 (43,96,198,263,264,338,339,347,427); depresses enzyme isocitric dehydrogenase (ICD) in fetus, causes reduced iodine uptake, autoimmune thyroiditis, & hypothyroidism.
(50,91,212,222,369,382,459,35). According to survey tests, 8 to 10 % of untreated women were found to have thyroid imbalances so the actual level of hypothyroidism is higher than commonly recognized(508). Even larger percentages of women had elevated levels of antithyroglobulin(anti-TG) or antithyroid peroxidase antibody(anti-TP). Studies indicate that slight imbalances (subclinical) of thyroid hormones in expectant mothers can cause permanent neuropsychiatric damage in the developing fetus(509).
Low first trimester levels of free T4 and positive levels of anti-TP antibodies in the mother during pregnancy have been found to result significantly reduces IQs(509a-e) and causes psychomotor deficits(509f). Hypothyroidism is a well documented cause of mental retardation(511f). Women with the highest levels of thyroid-stimulating-hormone (TSH) and lowest free levels of thyroxine 17 weeks into their pregnancies were significantly more likely to have children who tested at least one standard deviation below normal on an IQ test taken at age 8(509a). Based on study findings, maternal hypothyroidism appears to play a role in at least 15% of children whose IQs are more than 1 standard deviation below the mean, millions of children..Studies have also established a connection between maternal thyroid disease and babies born with heart defects(509h). The American Assoc. of Clinical Endocrinologists advises that all women considering becoming pregnant should get a serum thyrotropin test so that hypothyroidism can be diagnosed and treated early(558). Another test that should be considered is a hair element test for mercury or toxic metal exposures and essential mineral imbalances.
Studies have also established a "clear association" between the presence of thyroid antibodies and spontaneous abortions(511). Levels of recurrent abortions in a population with positive levels of thyroid antibodies in one study were 40%, 5 times the normal rate(511). Hypothyroidism is a well documented risk factor in spontaneous abortions and infertility(9,511). Another study of pregnant women who suffer from hypothyroidism (underactive thyroid) found a four-times greater risk for miscarriage during the second trimester than those who don't(511), and women with untreated thyroid deficiency were four-times more likely to have a child with a developmental disabilities(509f-h). Mercury through its affects on the endocrine system is also documented to cause other reproductive effects including infertility, low sperm counts, abnormal sperm, endometriosis, PMS, adverse effects on reproductive organs, etc. (9,50,104,105,390,500).
Mercury blocks thyroid hormone production by occupying iodine binding sites and inhibiting hormone action even when the measured thyroid level appears to be in proper range(390,35). The thyroid and hypothalamus regulate body temperature and many metabolic processes including enzymatic processes that when inhibited result in higher dental decay(35) . Mercury damage thus commonly results in poor bodily temperature control, in addition to many problems caused by hormonal imbalances such as depression.
Such hormonal secretions are affected at levels of mercury exposure much lower than the acute toxicity effects normally tested (50,390,84), as previously confirmed by hormonal/reproductive problems in animal populations (104,381c,50d). Mercury also damages the blood brain barrier and facilitates penetration of the brain by other toxic metals and substances(311). Hypothyroidism is also known to be a major factor in cardiovascular disease(510,509h).
The pituitary gland controls many of the body's endocrine system functions and secretes hormones that control most bodily processes, including the immune system and reproductive systems . One study found mercury levels in the pituitary gland ranged from 6.3 to 77 ppb(85), while another(348) found the mean level to be 30ppb- levels found to be neurotoxic and cytotoxic in animal studies. Some of the effect on depression is related to mercury's effect of reducing the level of posterior pituitary hormone(oxytocin). Low levels of pituitary function are associated with depression and suicidal thoughts, and appear to be a major factor in suicide of teenagers and other vulnerable groups.
The pituitary glands of a group of dentists had 800 times more mercury than controls(99). This may explain why dentists have much higher levels of emotional problems, depression, suicide, etc(500,Section VIII.). Amalgam fillings, nickel and gold crowns are major factors in reducing pituitary function(35,50,369,etc.). Supplementary oxytocin extract has been found to alleviate many of these mood problems(35), along with replacement of metals in the mouth(107,500-Section VI.). The normalization of pituitary function also often normalizes menstrual cycle problems, endometriosis, and increases fertility(35,9,500).
Mercury accumulates in the adrenal gland and disrupts adrenal gland function(84,369,381).
In general immune activation from toxics such as heavy metals resulting in cytokine release and abnormalities of the hypothalamus-pituitary-adrenal axis can cause changes in the brain, fatigue, and severe psychological symptoms(369,375,379-383,453,107) such as depression, profound fatigue, muscoskeletal pain, sleep disturbances, gastrointestinal and neurological problems as are seen in CFS, Fibromyalgia, and autoimmune thyroiditis. Such symptoms usually improve significantly after amalgam removal(500,Section VI). Such hypersensitivity has been found most common in those with genetic predisposition to heavy metal sensitivity (342,369,375,382) such as found more frequently in patients with HLA-DRA antigens(375,381,383). A significant portions of the population appear to fall in this category.
Thyroid imbalances, which are documented to be commonly caused by mercury (369,382,459,35,50,91,212), have been found to play a major role in chronic heart conditions such as clogged arteries, mycardial infarction, and chronic heart failure(510). In a recent study, published in the Annals of Internal Medicine, researchers reported that subclinical hypothyroidism is highly prevalent in elderly women and is strongly and independently associated with cardiac atherosclerosis and myocardial infarction(510c).
People who tested hypothyroid usually have significantly higher levels of homocysteine and cholesterol, which are documented factors in heart disease. 50% of those testing hypothyroid, also had high levels of homocysteine (hyperhomocysteinenic) and 90% were either hyperhomocystemic or hypercholesterolemic(510a). These are also known factors in developing arteriosclerotic vascular disease. Homocysteine levels are significantly increased in hypothtyroid patients and normalize with treatment(510efg).
The thymus gland plays a significant part in the establishment of the immune system and lymphatic system from the 12th week of gestation until puberty. Inhibition of thymus function can thus affect proper development of the immune and lymphatic systems. Lymphocyte differentiation, maturation and peripheral functions are affected by the thymic protein hormone thymulin. Mercury at very low concentrations has been seen to impair some lymphocytic functions causing subclinical manifestations in exposed workers. Animal studies have shown mercury significantly inhibits thymulin production at very low micromolar levels of exposure(513a). The metal allergens mercuric chloride and nickel sulfate were found to stimulate DNA synthesis of both immature and mature thymocytes at low levels of exposure, so chronic exposure can have long term effects(513b).
Also, micromolar levels of mercuric ions specifically blocked synthesis of ribosomal RNA, causing fibrillarin relocation from the nucleolus to the nucleoplasm in epithelial cells as a consequence of the blockade of ribosomal RNA synthesis. This appears to be a factor in deregulation of basic cellular events and in autoimmunity caused by mercury. There were specific immunotoxic and biochemical alterations in lymphoid organs of mice treated at the lower doses of mercury. The immunological defects were consistent with altered T-cell function as evidenced by decreases in both T-cell mitogen and mixed leukocyte responses. There was a particular association between the T-cell defects and inhibition of thymic pyruvate kinase, the rate-limiting enzyme for glycolysis(513c). Pyruvate and glycolysis problems are often seen in mercury toxic children being treated for Autism (409).
A direct mechanism involving mercury's inhibition of hormones and cellular enzymatic processes by binding with the hydroxyl radical(SH) in amino acids appears to be a major part of the connection to allergic/immune reactive/autoimmune conditions such as autism/ADHD (409-411,439,464,468,476,33,160), schizophrenia(409,410), lupus(113,234,330,331,468), Scleroderma(468), eczema and psoriasis (323,375,385,419,33), and allergies (271,313,330,331, 369,375,468). Mercury and other toxic metals also form inorganic compounds with OH, NH2, CL, in addition to the SH radical and thus inhibits many cellular enzyme processes, coenzymes, hormones, and blood cells(405,409,500,555).
For example mercury has been found to strongly inhibit the activity of dipeptyl peptidase (DPP IV) which is required in the digestion of the milk protein casein(411,412) as well as of xanthine oxidase(439). Studies involving a large sample of autistic and schizophrenic patients found that over 90 % of those tested had high levels of the neurotoxic milk protein beta-casomorphine-7 in their blood and urine and defective enzymatic processes for digesting milk protein(410).
Elimination of milk products from the diet has been found to improve the condition. Similar results have been seen in similarly but lesser affected patients with other pervasive developmental conditions such as ADHD. Such populations have also been found to have many with high levels of mercury who recover after mercury detox (409,413,369,160). As mercury levels are reduced the protein binding is reduced and improvement in the enzymatic process occurs. Additional cellular level enzymatic effects of mercury's binding with proteins include blockage of sulfur oxidation processes (33,114,194,330,331,412), enzymatic processes involving vitamins B6 and B12(418), effects on the cytochrome-C energy processes (43,84,338c,35), along with mercury's adverse effects on cellular mineral levels of calcium, magnesium, zinc, and lithium (43,96,333,338,160,500). Thus some of the main mechanisms of toxic effects of metals include cytotoxicity; changes in cellular membrane permeability; inhibition of enzymes, coenzymes, and hormones; and generation of lipid peroxides or free radicals- which result in neurotoxicity, immunotoxicity, impaired cellular respiration, gastrointestinal/metabolic effects, hormonal effects, and immune reactivity or autoimmunity.
Mercury has been found to cause hormonal changes which cause hair loss and greying of hair. In a large German study where 20,000 were tested, allergies and hair-loss were found to be 2-3 times as high in a group with large numbers of amalgam fillings compared to controls(199,9). Levels of mercury in follicular fluid was significantly higher for those with amalgam fillings (9,146). Based on this finding, a Gynecological Clinic that sees a large number of women suffering from alopecia/hair loss that was not responding to treatment had Amalgams replaced in 132 women who had not responded to treatment. 68 % of the women then responded to treatment and alopecia was alleviated(187). In other studies involving amalgam removal, the majority had significant improvement (40,317,500). Higher levels of hormone disturbances, immune disturbances, infertility, and recurrent fungal infections were also found in the amalgam group. The results of hormone tests, cell culture studies, an intervention studies agree(9,146).
Other clinics have also found alleviation of hair loss/alopecia after amalgam removal and detox(40,317). Another study in Japan found significantly higher levels of mercury in gray hair than in dark hair(402).
References
(2) United States Environmental Protection Agency, Office of Water, Novermber 2000, The National Listing of Fish and Wildlife Advisories:, EPA-823-F-00-20, http://www.epa.gov/ost/fish/advisories/general.html
& The Conference of New England Governors and Eastern Canadian Premiers, New England Governors/ Eastern Canadian Premiers Mercury Action Plan- 1998; http://www.cmp.ca/neg/reports/mercury.htm#anchor1 & U.S. EPA, FDA, Advisory on Fish Consumption by Women of Child Bearing Age and Children, http://www.epa.gov/mercury/fishadv.pdf
(9)(a) Dr.I.Gerhard, Dr. E.Roller,et al, Tubingen Univ. Gynecological Clinic, Heidelberg,1996; & (b)Gerhard I, Monga B, Waldbrenner A, Runnebaum B "Heavy Metals and Fertility", J of Toxicology and Environmental Health,Part A, 54(8):593-611, 1998; & (c) Gerhard I, Waibel S, Daniel V, Runnebaum B "Impact of heavy metals on hormonal and immunological factors in women with repeated miscarriages", Hum Reprod Update 1998 May;4(3):301-309; & (d) Gerhard I, "Ganzheitiche Diagnostik un Therapie bie Infertilitat", Erfahrungsheilkunde,1993, 42(3): 100-106; & (e)"Hormonal conditions affecting women caused by environmental poisons" in Pravention, Diagnose und Therapie von Umwelterkrankungen, JD Kruse-Jarres(Ed.), 1993, p51-68; & (f) Gerhard I, Waldbrenner P, Thuro H, Runnebaum B, Diagnosis of heavy metal loading by the oral DMPS and chewing gum tests. Klinisches Labor 1992, 38:404-411.
(14) (a).Nylander, Weiner JA, "Mercury concentrations in the human brain and kidneys and exposure from amalgam fillings", Swed Dent J 1987; 11:179-187, & Prosth Dent 1987, 58:704-707; &(b) Schupp, Riedel et al, " the mercury concentration in humans from amalgam fillings", Organen.Dt.Zahnarztl.Z. 1992; 47:490-496; & (c) D.W.Eggleston et al, Correlation of dental amalgam with mercury in brain tissue. J Prosthet Dent, 1987,58(6),704-7.
(16) Lichtenberg H, "Mercury vapor in the oral cavity in relation to number of amalgam surfaces and the classic symptoms of chronic mercury poisoning", J Orthomol Med (1996), v11, n.2, 87-94
(19)(a) Matts Hanson. Dept of Zoophysiology, University of Lund, Sweden. "Amalgam hazards in your teeth", J. Orthomolecular Psychiatry 1983; 2(3): 194-201; & (b) .Lorscheider FL, Vimy MJ, "Evaluation of the safety issue of mercury release from amalgam fillings", 1993, FASEB J, 7:1432-33.
(20)(a) Vimy MJ, Takahashi,Y, Lorscheider FL; Maternal -Fetal Distribution of Mercury Released From Dental Amalgam Fillings. Dept of Medicine and Medical Physiology , faculty of Medicine, Univ. of Calgary, Calgary Alberta Canada, 1990 & Amer.J.Physiol.,1990, 258:R939-945; & (b) Hahn LJ, Kloiber R, Leininger RW, Vimy MJ, Lorscheider FL. Distribution of mercury released from amalgam fillings into monkey tissues", FASEB J.,1990, 4:5536
(21) R.A.Goyer,"Toxic effects of metals"in: Caserett and Doull's Toxicology- TheBasic Science of Poisons, McGraw-Hill Inc., N.Y., 1993; &(b) Goodman, Gillman, The Pharmacological Basis of Therapeutics, Mac Millan Publishing Company, N.Y. 1985; &(c) Encyclopedia of Occumpational Health and Safety, International Labour Office, Geneva, Vol 2, 3rd Edition.
(33) S.C. Langley-Evans et al, "SO2: a potent glutathione depleting agent", Comp Biochem Physiol Pharmocol Toxicol Endocrinol, 114(2):89-98; & (b)P.E. Emory et al, "Increased Prevalence of poor sulphuoxidation in patients with Rheumatoid Arthritis", Ann Rheum Dis, 1992, 51(3): 318-20; & © Markovich et al, "Heavy metals (Hg,Cd) inhibit the activity of the liver and kidney sulfate transporter Sat-1", Toxicol Appl Pharmacol, 1999,154(2):181-7; & (d)S.A. McFadden, "Xenobiotic metabolism and adverse environmental response: sulfur-dependent detox pathways",Toxicology, 1996, 111(1-3):43-65; & (e)Alberti A, Pirrone P, Elia M, Waring RH, Romano C. Sulphation deficit in "low-functioning" autistic children. Biol Psychiatry 1999, 46(3):420-4.
(35) Huggins HA, Levy,TE, Uniformed Consent: the hidden dangers in dental care, 1999, Hampton Roads Publishing Company Inc; & Hal Huggins, Its All in Your Head, 1997; & Center for Progressive Medicine, 1999, http://www.hugnet.com/
(38) S.Ziff and M.Ziff, Infertility and Birth Defects: Is Mercury from Dental Fillings a Hidden Cause?, Bio-Probe, Inc. ISBN: 0-941011-03-8.1987
(43) (a)Knapp LT; Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem 2000 May 22; & (b)B.Rajanna et al, "Modulation of protein kinase C by heavy metals", Toxicol Lett, 1995, 81(2-3):197-203 .
(49) A.Kingman et al, National Institute of Dental Research, "Mercury concentrations in urine and blood associated with amalgam exposure in the U.S. military population", Dent Res, 1998, 77(3):461-71.
(50) Sin YM, Teh WF, Wong MK, Reddy PK - "Effect of Mercury on Glutathione and Thyroid Hormones" Bulletin of Environmental Contamination and Toxicology 44(4):616-622 (1990); & (b) J.Kawada et al, "Effects of inorganic and methyl mercury on thyroidal function", J Pharmacobiodyn, 1980, 3(3):149-59; & (c) Ghosh N. Thyrotoxicity of cadmium and mercury. Biomed Environ Sci 1992, 5(3): 236-40; &(d) Goldman, Blackburn, The Effect of Mercuric Chloride on Thyroid Function of the Rat, Toxicol and Applied Pharm 1979, 48: 49-55; &(e)Kabuto M - "Chronic effects of methylmercury on the urinary excretion of catecholamines and their responses to hypoglycemic stress" Arch Toxicol 65(2):164-7 (1991) ; & Assoc. for Birth Defect Children, Birth Defect News, March 2001; (? add topic title)
(61) (a)E.Lutz et al, "Concentrations of mercury in brain and kidney of fetuses and infants", Journal of Trace Elements in Medicine and Biology, 1996,10:61-67; & (b)G.Drasch et al, "Mercury Burden of Human Fetal and Infant Tissues", Eur J Pediatr 153:607-610,1994; ?
(84) (a)J.C.Veltman et al, "Alterations of heme, cytochrome P-450, and steroid metabolism by mercury in rat adrenal gland", Arch Biochem Biophys, 1986, 248(2):467-78; &(b) A.G.Riedl et al, Neurodegenerative Disease Research Center, King's College, UK, "P450 and hemeoxygenase enzymes in the basal ganglia and their role's in Parkinson's disease", Adv Neurol, 1999; 80:271-86; &(c) Alfred V. Zamm. Dental Mercury: A Factor that Aggravates and Induces Xenobiotic Intolerance. J. Orthmol. Med. v6#2 pp67-77 (1991).
(85) Weiner JA, Nylander M; The relationship between mercury concentration in human organs and different predictor variables. Sci Total Environ 1993 Sep 30;138(1-3):101-15 ; & (b)Weiner JA, Nylander M. An estimation of the uptake of mercury from amalgam fillings based on urinary excretion of mercury in Swedish subjects. Sci Total Environ 1995 30;168(3):255-265
(91) B.Lindqvist et al, "Effects of removing amalgam fillings from patients with diseases affecting the immune system", Med Sci Res 24(5): 355-356, 1996. ?
(96) A.F.Goldberg et al, "Effect of Amalgam restorations on whole body potassium and bone mineral content in older men",Gen Dent, 1996, 44(3): 246-8; & (b) K.Schirrmacher,1998, "Effects of lead, mercury, and methyl mercury on gap junctions and [Ca2+]I in bone cells", Calcif Tissue Int 1998 Aug;63(2):134-9. ?
(99) M. Nylander et al, Mercury accumulation in tissues from dental staff and controls", Swedish Dental Journal, 13:235-243, 1989; & (b) M. NYLANDER et al, Br J Ind Med 1991, 48(11):729-34; & (c) Nylander M, "Mercury in pituitary glands of dentists", Lancet,442, Feb 26, 1986.
(104) (a)C.F.Facemire et al, "Reproductive impairment in the Florida Panther", Health Perspect,1995, 103 (Supp4):79-86; &(b) J.M.Yang et al, "The distribution of HgCl2 in rat body and its effect on fetus", Environ Sci , 1996, 9(4): 437-42;(c) M.Maretta et al, "Effect of mercury on the epithelium of the fowl testis", Vet Hung 1995, 43(1):153-6. ??
(105) (a)T.Colborn(Ed.),Chemically Induced Alterations in Functional Development, Princeton Scientific Press,1992; &(b) Colborn T, " Developmental Effects of Endocrine-Disrupting Chemicals",Environ Heath Perspectives, V 101, No.5, Oct 1993; & (c)B.Windham, "Health, Hormonal, and Reproductive Effects of Endocrine Disrupting Chemicals" (including mercury), Annotated Bibliography ,2000; &(d) Giwercman A, Carlsen E, Keiding N, Skakkabaek NE, Evidence for increasing incidence of abnormalities of the human testis: a review. Environ Health Perspect 1993; 101 Suppl(2): 65-71.
(107) R.L.Siblerud et al,"Psychometric evidence that mercury from dental fillings may be a factor in depression,anger,and anxiety", Psychol Rep, v74,n1,1994; & Amer. J. Of Psychotherapy, 1989; 58: 575-87; Poisoning and Toxicology compendium,Leikin & Palouchek, Lexi-Comp,1998,p705. ?
(113) (a)T.A.Glavinskiaia et al, "Complexons in the treatment of lupus erghematousus", Dermatol Venerol, 1980, 12: 24-28; & (b)A.F.Hall, Arch Dermatol 47, 1943, 610-611. ?
(114) (a)M.Aschner et al, "Metallothionein induction in fetal rat brain by in utero exposure to elemental mercury
vapor", Brain Research, 1997, dec 5, 778(1):222-32; & (b) O'Halloran TV, "Transition metals in control
Of gene expression", Science, 1993, 261(5122):715-25; & (c)Matts RL, Schatz JR, Hurst R, Kagen R. Toxic heavy metal ions inhibit reduction of disulfide bonds. J Biol Chem 1991; 266(19): 12695-702; & (d) Boot JH. Effects of SH-blocking compounds on the energy metabolism in isolated rat hepatocytes. Cell Struct Funct 1995; 20(3): 233-8.
(122) B.Ono et al, "Reduced tyrosine uptake in strains sensitive to inorganic mercury", Genet, 1987,11(5):399-
(146) (a) Gerhard I, Runnebaum B, The limits of hormone substitution in pollutant exposure and fertility disorders Zentralbl Gynakol, 1992, 114, 593-602: &(b)Gerhard, I.: Fortpflanzungsstörungen durch Umweltgifte? Therapeutikon 7, 478-491 (1993).; &(c)Roller, E., Vallon, U. und Clédon, Ph.: Einfluß von Schwermetallen auf die Progesteronsynthese von Leydig-Zellen. J Fert Reprod 3, 33 (1995). &(d) Vallon U, Roller E, und Clédon, Ph.: Schwermetallionen beeinflussen die Progesteronsynthese von humanen Granulosazellen bei IVF-Patientinnen: Anwendung eines alternativen in-vitro-Zytotoxizitätstests. J Fert Reprod 3, 31 (1995).
(160) B. Windham, Cognitive and Behavioral Effects of Toxic Metals, 2001. (over 150 medical study references)
http://www.home.earthlink.net/~berniew/tmlbn.html
(181) Mathieson PW, "Mercury: god of TH2 cells",1995, Clinical Exp Immunol.,102(2):229-30; & Heo Y, Parsons PJ, Lawrence DA, Lead differentially modifies cytokine production in vitro and in vivo. Toxicol Appl Pharmacol, 196; 138:149-57; & Murdoch RD, Pepys J; Enhancement of antibody and IgE production by mercury and platinum salts. Int Arch Allergy Appl Immunol 1986 80: 405-11.
(187) (a)Klobusch J, Rabe T, Gerhard I, Runnebaum B, "Alopecia and environmental pollution" Klinisches Labor 1992, 38:469- 476; & (b)"Schwermetallbelastungen bei Patientinnen mit Alopezie" Arch Gynecol. Obstet., 1993,254(1-4):278-80;& (c)G. Kunzel et al, "Arch Gynecol. Obstet., 1993, 254:277-8. ??
(192) (a) N.Nogi, "Electric current around dental metals as a factor producing allergic metal ions in the oral cavity", Nippon Hifuka Gakkai Zasshi, 1989, 99(12):1243-54; &(b) J. Bergdahl, A.J.Certosimo et al, National Naval Dental Center, "Oral Electricity", Gen Dent, 1996, 44(4):324-6; & (c)R.D.Meyer et al, "Intraoral galvanic corrosion",Prosthet Dent, 1993,69(2):141-3; & (d)B.M.Owens et al, "Localized galvanic shock after insertion of an amalgam restoration", Compendium, 1993, 14(10),1302,1304,1306-7. ??
(199) Dr. P.Kraub & M.Deyhle, Universitat Tubingen- Institut fur Organische Chemie, "Field Study on the Mercury Content of Saliva", 1997
http://www.uni-tuebingen.de/KRAUSS/amalgam.html
(20,000 people tested for mercury level in saliva and health status/symptoms compiled)
(211) M.J.Vimy and F.L. Lorscheider, Faculty of Medicine, Univ. Of Calgary, July 1991. J. Trace Elem. Exper. Med., 1990,3, 111-123.
(217) Agency for Toxic Substances and Disease Registry, U.S. Public Health Service, Toxicological Profile for Mercury , 1999; & (b)Apr 19,1999 Media Advisory, New MRLs for toxic substances, MRL:elemental mercury vapor/inhalation/chronic & MRL: methyl mercury/ oral/acute; &
http://www.atsdr.cdc.gov/mrls.html
(234) P.E. Bigazzi, "Autoimmunity and Heavy Metals", Lupus, 1994; 3: 449-453;(b) & Pollard KM, Pearson Dl, Hultman P. Lupus-prone mice as model to study xenobiotic-induced autoimmunity. Environ Health Perspect 1999; 107(Suppl 5): 729-735; &(c) Nielsen JB; Hultman P. Experimental studies on genetically determined susceptibility to mercury-induced autoimmune response. Ren Fail 1999 May-Jul;21(3-4):343-8; &(d) Hultman P, Enestrom S, Mercury induced antinuclear antibodies in mice, Clinical and Exper Immunology, 1988, 71(2): 269-274.
(271) B.A.Weber, "The Marburg Amalgam Study", Arzt und Umwelt, Apr, 1995; (266 cases) & (b) "Amalgam and Allergy", Institute for Naturopathic Medicine, 1994; (40 MS cases), http://home,t-online.de/home/Institut_f._Naturheilverfahren/patinf.htm
(273) R.Schiele et al, Institute of Occupational Medicine, Univ. Of Erlamgem- Nurnberg, "Studies of organ mercury content related to number of amalgam fillings",Symposium paper, March 12, 1984, Cologne, Germany; (in 38); &(b) " Mercury Mobilization by DMPS in persons with and without amalgam
Fillings ", Zahnarztl. Mitt, 1989, 79(17): 1866-1868; &(c) J.J.Kleber, "Mercury in the urine after DMPS" in [Status Quo and Perspectives of Amalgam], L.T. Friberg(ed.), Georg-Thieme Verlag, Stuttgart, New York, 1005, p 61-69.
(274) L.Friberg et al, "Mercury in the brain and CNS in relation to amalgam fillings", Lakartidningen, 83(7):519-521,1986(Swedish Medical Journal)
(287) Warvinge K, Mercury distribution in the neonatal and adult cerebellum after mercury vapor exposure of pregnant squirrel monkeys, Environ Res 2000, 83(2): 93-101; & (b)M.C. Newland et al,"Behavioral consequences of in utero exposure to mercury vapor in squirrel monkeys", Toxicology & Applied Pharmacology, 1996, 139: 374-386.
(296) (a) L.Bucio et al, Uptake, cellular distribution and DNA damage produced by mercuric chloride in a human fetal hepatic cell line. Mutat Res 1999 Jan 25;423(1-2):65-72; & (b)Snyder RD; Lachmann PJ; Thiol involvement in the inhibition of DNA repair by metals in mammalian cells. Source Mol Toxicol, 1989 Apr-Jun, 2:2, 117-28 & (c) L.Verschaeve et al, "Comparative in vitro cytogenetic studies in mercury-exposed human lymphocytes", Muta Res, 1985, 157(2-3):221-6; & (d) L.Verschaeve,"Genetic damage induced by low level mercury exposure", Envir Res,12:306-10,1976: ?
(317) S.Zinecker, "Amalgam: inorganic mercury in the brain", der Kassenarzt, 1992, 32(4):23; "Praxiproblem Amalgam", Der Allgermeinarzt, 1995,17(11):1215-1221. (1800 patients)
(323) (a)Dr. Kohdera, Faculty of Dentistry, Osaka Univ., International Congress of Allergology and Clinical Immunology, EAACI, Stockholm, June 1994; & Heavy Metal Bulletin, Vol 1, Issue 2, Oct 1994. (160 cases cured-eczema); (b) Tsunetoshi Kohdera, MD, dermatology, allergology, 31 Higashitakada-cho Mibu Nakagyo-ku Schimazu Clinics Kyoto 604 Japan e-mail:smc-inet@mbox.kyoto-inet.or.jp &(c) P.Dallmann,"Dermatalogical conditions caused by amalgam? PeDa_Eigenverisg, 1995; & (d)G. Ionescu, Biol Med, 1996, (2): 65-68; & (e) Ionescu G.: Tooth alloys. Electro-chemical and biological processes.
Materialprueuefung.. Komplementaeaermed. , 3, 72-77, 1996; & (f) Ionescu G; Heavy metal load by Dental materials. Experience with Neurodermitis and Psoriasis patients.. Zeitung f. Umweltmedizin, 3, 163-171, 1997 ?
(327) Danscher G; Horsted-Bindslev P; Rungby J. Traces of mercury in organs from primates with amalgam fillings. Exp Mol Pathol 1990;52(3):291-9; ??
(330) (a) C.M. Tanner et al,"Abnormal Liver Enzyme Metabolism in Parkinson's",Neurology, 1991, 41(5): Suppl 2, 89-92; & (b) M.Watanabe et al, Amino Acids, 1998, 15(2): 143-50 & (c) M.T.Heafield et al, "Plasma cysteine and sulphate levels in patients with Motor neurone disease, Parkinson's Disease, and Alzheimer's's Disease", Neurosci Lett, 1990, 110(1-2), 216,20; & (d) A.Pean et al, "Pathways of cysteine metabolism in MND/ALS", J neurol Sci, 1994, 124, Suppl:59-61. ??
(331) (a)C.Gordon et al, "Abnormal sulphur oxidation in systemic lupus erythrmatosus(SLE)", Lancet, 1992,339:8784,25-6; &(d) P.Emory et al, "Poor sulphoxidation in patients with rheumatoid arthritis", Ann Rheum Dis, 1992, 51:3,318-20; & (c)P.Emory et al, Br J Rheumotol, 1992, 31:7,449-51; &(d) Steventon GB, et al; Xenobiotic metabolism in motor neuron disease, The Lancet, Sept 17 1988, p 644-47; & Neurology 1990, 40:1095-98. ??
(333) (a)A.J.Freitas et al, "Effects of Hg2+ and CH3Hg+ on Ca2+ fluxes in the rat brain", Brain Research, 1996, 738(2): 257-64; & (b)P.R.Yallapragoda et al,"Inhibition of calcium transport by Hg salts" in rat cerebellum and cerebral cortex", J Appl toxicol, 1996, 164(4): 325-30; & (c) E.Chavez et al, "Mitochondrial calcium release by Hg+2",J Biol Chem, 1988, 263:8, 3582-;&(d) A. Szucs et al, Cell Mol Neurobiol, 1997,17(3): 273-8; & (e) D.Busselberg, 1995, "Calcium channels as target sites of heavy metals",Toxicol Lett, Dec;82-83:255-61; & (f) Cell Mol Neurobiol 1994 Dec;14(6):675-87; ?? ??
(337) H.G. Abadin, et al, U.S. ATSDR, "Breast-feeding exposure of infants to mercury, lead, and cadmium: A Public Health Perspective", Toxicol Ind Health, 1997, 13(4): 495-517. ?
(338) (a)W.Y.Boadi et al, Dept. Of Food Engineering and Biotechnology, T-I Inst of Tech., Haifa, Israel, "In vitro effect of mercury on enzyme activities and its accumulation in the first-trimester human placenta", Environ Res, 1992, 57(1):96-106;& (b) "In vitro exposure to mercury and cadmium alters term human placental membrane fluidity", Pharmacol, 1992, 116(1): 17-23; & (c)J.Urbach et al, Dept. of Obstetrics & Gynecology, Rambam Medical Center, Haifa, Israel, "Effect of inorganic mercury on in vitro placental nutrient transfer and oxygen consumption", Reprod Toxicol, 1992,6(1):69-75;& (d) Karp W, Gale TF et al, Effect of mercuric acetate on selected enzymes of maternal and fetal hamsters" Environmental Research, 36:351-358 ??
(342) Stejskal VDM, Danersund A, Lindvall A, Hudecek R, Nordman V, Yaqob A et al. Metal- specific memory lymphocytes: biomarkers of sensitivity in man. Neuroendocrinology Letters, 1999; 20: 289-98.
(348)(a) Kistner A, "Mercury poisoning by amalgam: Diagnosis and therapy" ZWR, 1995,104(5):412-417; &(b) Mass C, Bruck W. "Study on the significance of mercury accumulation in the brain from dental amalgam fillings through direct mouth-nose-brain transport", Zentralbl Hyg Umweltmed 1996; 198(3): 275-91.
(363) J.W.Reinhardt, Univ. Of Iowa College of Dentistry, "Side effects: mercury contribution to
body burden from dental amalgam", Adv Dent Res, 1992, 6: 110-3.
(366) (a)"Tooth amalgam and pregnancy", Geburtshilfe Frauenheikd. 1995, 55(6): M63-M65; &(b) T. Zinke, "There are new realizations to the Amalgam problem", in Status Quo and perspectiveves of Amalgam and Other Dental Materials, L.F. Friberg(Ed.), Georg=Thieme-Verlag, Stuttgart, New York, 1995, p1-7. ?
(367)(a) Gerhard I, "Amalgam from gynacological view", Der Frauenarzt, 1995,36(6): 627-28; & (b)"Schdstoffe und Fertillitatsstorungen", Schwermetalle und Mineralstoffe, Geburtshilfe Frauenheikd, 1992, 52(7):383-396; & (c) Gerhard I, "Reproductive risks of heavy metals and pesticides in women", in: Reproductive Toxicology, M.Richardson(ed.), VCH Weinhelm, 1993, 167-83; & (d)Gerhard I, "Infertility with women by environmental illnesses, JD. Kruse-Jarres(Ed.), 1993, 51-68.
(369) Sterzl I, Prochazkova J, Stejskal VDM et al, Mercury and nickel allergy: risk factors in fatigue and autoimmunity. Neuroendocrinology Letters 1999; 20:221-228. http://www.melisa.org/
(372) (a)Atchison WD. Effects of neurotoxicants on synaptic transmission. Neurotoxicol Teratol 1998, 10(5):393- 416; & Sidransky H, Verney E, Influence of lead acetate and selected metal salts on tryptophan binding to rat hepatic nuclei. Toxicol Pathol 1999, 27(4):441-7; &(b) Shukla GS, Chandra SV, Effect of interaction of Mn2+withZn2+, Hg2+, and Cd2+ on some neurochemicals in rats. Toxicol Lett 1982, 10(2-3):163-8; &(c)Brouwer M et al, Functional changes induced by heavy metal ions. Biochemistry, 1982, 21(20): 2529-38.
(375) (a) Stejskal VDM, Danersund A, Lindvall A. Metal-specific memory lymphocytes: biomarkers of sensitivity in man. Neuroendocrinology Letters 1999; &(b) Stejskal V, Hudecek R, Mayer W, "Metal-specific lymphocytes: risk factors in CFS and other related diseases", Neuroendocrinology Letters, 20: 289-298, 1999
http://www.melisa.org/
(379)(a) MacDonald EM, Mann AH, Thomas HC. Interferons as mediators of psychiatric morbidity. The Lancet 1978; Nov 21, 1175-78; & (b) Hickie I, Lloyd A. Are cytokines associated with neuropsychiatric syndrome in humans? Int J Immunopharm 1995; 4:285-294.
(380) (a) Komaroff AL, Buchwald DS. Chronic fatigue syndrom: an update. Ann Rev Med 1998; 49: 1-13; &
(b) Buchwald DS, Wener MH, Kith P. Markers of inflamation and immune activation in CFS. J Rheumatol 1997; 24:372-76.
(381) (a) Demitrack MA, Dale JK. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metabol 1991; 73:1224-1234; & (b)Turnbull AV, Rivier C. Regulation of the HPA axis by cytokines. Brain Behav Immun 1995; 20:253-75; & (c)Ng TB, Liu WK. In Vitro Cell Dev Biol 1990 Jan;26(1):24-8. Toxic effect of heavy metals on cells isolated from the rat adrenal and testis.
(382) Sterzl I, Fucikova T, Zamrazil V. The fatigue syndrome in autoimmune thyroiditis with polyglandular activation of autoimmunity. Vnitrni Lekarstvi 1998; 44: 456-60. http://www.melisa.org/ ;
&(b) Sterzl I, Hrda P, Prochazkova J, Bartova J, Reactions to metals in patients with chronic fatigue and autoimmune endocrinopathy. Vnitr Lek 1999 Sep;45(9):527-31 ; & & (c)Kolenic J, Palcakova D, Benicky L, Kolenicova M - "The frequency of auto-antibody occurrence in occupational risk (mercury)" Prac Lek 45(2):75-77 (1993)
(383)(a) Saito K. Analysis of a genetic factor of metal allergy-polymorphism of HLA-DR-DO gene. Kokubyo Gakkai Zasschi 1996; 63: 53-69; &(b) Prochazkova J, Ivaskova E, Bartova J, Stejskal VDM. Immunogentic findings in patients with altered tolerance to heavy metals. Eur J Human Genet 1998; 6: 175.
(385)(a) Kohdera T, Koh N, Koh R. Antigen-specific lymphocyte stimulation test on patients with psoriasis vulgaris. XVI International Congress of Allergology and Clinical Immunology, Oct 1997, Cancoon, Mexico; & (b)Ionescu G,. Heavy metal load with atopic Dermatitis and Psoriasis, Biol Med 1996; 2:65-68; &
(c) A subset of patients with common variable immunodeficiency. Blood 1993, 82(1): 192-20.
(387) Caulk, Inc. (amalgam manufacturer),Material Data Safety Sheet,1997, (provided to dentists)
http://www.caulk.com/mSDSDFU/DISPERSDFU.html
(390) (a)Ellingsen DG, Nordhagen HP, Thomassen Y. Uninary selenium excretion in workers with low exposure to mercury vapor. J Appl toxicol 1995; 15(1): 33-6; &(b) Ellingsen DG, Efskind J, Haug E, Thomassen Y, Martinsen I, Gaarder PI - "Effects of low mercury vapour exposure on the thyroid function in chloralkali workers" J Appl Toxicol 20(6):483-9 (2000)http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?uid=11180271&form=6&db=m&Dopt=r &(c) Barregard L, Lindstedt G, Schutz A, Sallsten G - "Endocrine function in mercury exposed chloralkali workers" Occup Environ Med 51(8):536-40 (1994)
http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?uid=7951778&form=6&db=m&Dopt=r
Watanabe C, Yoshida K, Kasanuma Y, Kun Y, Satoh H. In utero methylmercury exposure differentially affects the activities of selenoenzymes in the fetal mouse brain.Environ Res 1999 Apr;80(3):208-14.
(402) Ando T, Wakisaka I, Hatano H. Mercury concentration in gray hair. Nippon Eiseigaku Zasshi 1989; 43(6):1063-8.
(405) Jenny Stejskal, Vera Stejskal. The role of metals in autoimmune diseases and the link to neuroendocrinology Neuroendocrinology Letters, 20:345-358, 1999.
(409) (a)Autism: a unique form of mercury poisoning.
http://www.autism.com/ari/mercurylong.html;
& (b) Yazbak FE(MD,FAAP) Autism 99: A National Emergency, &http://www.garynull.com/documents/autism_99.htm;
& (c) Dr. A Holmes, Autism Treatment Center,Baton Rouge, La,
http://www.healing-arts.org/children/holmes.htm;
& (c)Jaquelyn McCandless, M.D., Autism Spectrum Treatment Center, Woodland Hills, Ca http://www.home.earthlink.net/~berniew1/kidshg.html
(410) J.R. Cade et al, Autism and schizophrenia linked to malfunctioning enzyme for milk protein digestion. Autism, Mar 1999.
(411) (a) Puschel G, Mentlein R, Heymann E, 'Isolation and characterization of dipeptyl peptidase IV from human placenta', Eur J Biochem 1982 Aug;126(2):359-65; &(b) Kar NC, Pearson cm. Dipeptyl Peptidases in human muscle disease. Clin Chim Acta 1978; 82(1-2): 185-92; &(c) Seroussi K, Autism and Pervasive Developmental Disorders , 1998, p174, etc.
(412) (c) Moreno-Fuenmayor H, Borjas L, Arrieta A, Valera V, Plasma excitatory amino acids in autism. Invest Clin 1996,37(2): 113-28; & (b)Rolf LH, Haarman FY, Grotemeyer KH, Kehrer H. Serotonin and amino acid content in platelets of autistic children. Acta Psychiatr Scand 1993, 87(5): 312-6; & (c)Naruse H, Hayashi T, Takesada M, Yamazaki K. Metabolic changes in aromatic amino acids and monoamines in infantile autism and a new related treatment, No To Hattatsu, 1989, 21(2):181-9; &(d) Carlsson ML. Is infantile autism a hypoglutamatergic disorder? J Neural Transm 1998, 105(4-5): 525-35.
(413) (a) Edelson SB, Cantor DS. Autism: xenobiotic influences. Toxicol Ind Health 1998; 14(4): 553-63; & (b) Liska, DJ. The detoxification enzyme systems. Altern Med Rev 1998. 3(3):187-98; & (c)
http://www.edelesoncenter.com/
(418) Srikantaiah MV; Radhakrishnan AN. Studies on the metabolism of vitamin B6 in the small intestine. Purification and properties of monkey intestinal pyridoxal kinase. Indian J Biochem 1970 Sep;7(3):151-6. ?
(419) (a) Lipozencic J; Milavec-Puretic V; Pasic A. Contact allergy and psoriasis. Arh Hig Rada Toksikol 1992 Sep;43(3):249-54; & (b) Roujeau JC et al, Acute generalized exanthematous pustulosis. Analysis of 63 cases; Arch Dermatol 1991 Sep;127(9):1333-8 ?
(439) (a) Mercuric chloride intoxication. Part 1, Bull Environ Contam Toxicol 1978; 20(6): 729-35; & (b) Mondal MS, Mitra S. Inhibition of bovine xanthine oxidase activity by Hg2+ and other metal ions. J Inorg Biochem 1996; 62(4): 271-9; & (c) Sastry KV, Gupta PK. In vitro inhibition of digestive enzymes by heavy metals and their reversal by chelating agents:
(453) Blumer W, "Mercury toxicity and dental amalgam fillings", Journal of Advancement in Medicine, v.11, n.3, Fall 1998, p.219
(458) Dowling AL, Iannacone EA, Zoeller RT. Maternal Hypothyroidism Selectively Affects the Expression of Neuroendocrine-Specific Protein A Messenger Ribonucleic Acid in the Proliferative Zone of the Fetal Rat Brain Cortex. Endocrinology 2001 Jan 1;142(1):390-399
(459) Isny Clinic(South Germany) Kurt Muller , MD, member of Editorial board for Ganzheitliches Medicine Journal. Wassertornstrasse 6 , Isny, BRD fax: 0049 7562 550 52
(464) (a) Walsh, WJ, Health Research Institute, Autism and Metal Metabolism, http://www.hriptc.org/autism.htm,
Oct 20, 2000; & (b) Walsh WJ, Pfeiffer Treatment Center, Metal-Metabolism and Human Functioning, 2000,
http://www.hriptc.org/mhfres.htm
(476) (a) Dr Thomas Verstraeten, US Centres for Disease Control and Prevention, Summary Results: Vaccine Safety Datalink Project - a database of 400,000 children , May 2000; & (b) Halsey, NA. Limiting Infant Exposure to Thimerosal in vaccines. J. of the Amer. Medical Assoc., 282: 1763-66; & (c) The Center for Biologics Evaluation and Research (CBER), Review of the Use of Thimerosal in Vaccines, The US Food and Drug Administration(FDA), Jul 4, 2000.
(500) B.Windham, Common Exposure Levels and Adverse Health Effects from Mercury/Amalgam Dental Fillings, and Results of Replacement of Amalgam Fillings, Review, 2001. (over 1500 peer-reviewed studies documenting common exposures more than Gov't health guidelines and mechinisms of causality of 40 chronic conditions, and 60,000 clinical cases of recovery or significant improvement after amalgam replacement as followed by doctors)http://www.home.earthlink.net/~berniew1/amalg6.html
(508)(a) Bonar DB, McColgan B, Smith DR, Darke C, Guttridge MG, Williams HSmyth PPA, Hypothyroidism and aging: The Rosses' Survey. Thyroid 2000, 10(9):821-827;& (b) Canaris GJ, Manowitz NR, Mayor G, Ridgway EC.
The Colorado thyroid disease prevalence study. Arch Tntern Med 2000, 160(4):526-34; &(c) GS Connection 11(12): Prevelence of Thyroid Imbalance, Thyroid in Pregnancy, GSDL, http://www.gsdl.com/
(509)(a) Klein RZ, Sargent JD, Larsen PR, Waisbren Se, Haddow JE, Mitchell ML, Relation of severity of maternal hypothyroidism to cognitive development of offspring. J Med Screen 2001: 8:18-20; &(b) de Escobar DM, Orbregon MF, del Rey FE, Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? C Clin Endocrin Metab 2000; 3975-3987; &(c) Thyroid Imbalances in Pregnancy Linked to Poor Child Neurodelopment, Great Smokies Diagnostic Lab, http://www.gsdl.com/news/connections/vol11/conn20010228.html
&(d) J. E. Haddow et al, Babies Born to Mothers with Untreated Hypothyroidism Have Lower I.Q.'s, New England Journal of Medicine, Aug 19, 1999; & (e) Lavado-Autric et al. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. JCI 111:1073-1082 (2003); & (f)Pop VJ, Vader HL et al, Low maternal free thyroxine during early pregnancy is associated with impaired psychomotor development in infancy, Clin Endocrinol(Oxf), 50:149-55, 1999; & Man EB, Brown JF, Serunian SA. Maternal hypothyroxinemia: psychoneurological deficits of progeny. Ann Clin Lab Sci 1991;21(4):227-39; & Pharoah POD, Connolly KJ et al, Maternal thyroid hormone levels in pregnancy and cognitive and motor performance of the children, Clin Endocrinol(Oxf), 1984, 21:265-70; & (g) Pop VJ, de Vries E, et al, Maternal thyroid peroxidase antibodies during pregnancy: and impaired child development, J Clin Endocrinol Metab., 1995, 80:3561-3566 & Connors MH, Styne DM, Neonatal athyreosis resulting from thyrotropin-binding inhibitory immonoglobulins, Pediatrics, 1986, 78:287-290; & (h) Asami T, Suzuki H, Effects of thyroid hormone deficiency on electrocardiogram findings of congenenitally hypothyroid neonates. Thyroid 11: 765-8, 2001; & Kumar R, Chaudhuri BN. Altered maternal thyroid function: fetal and neonatal heart cholesterol and phospholipids, .Indian J Physiol Pharmacol 1993 Jul;37(3):176-82
(510) (a)Morris MS, Bostom AG, Jacques PJ, Selhub J, Rosenberg IH, Hyperhomocysteinemia and hypercholesterolemia associated with hypothyroidism in the third U.S. National Health and Nutrition Examination Survey, Artherosclerosis 2001, 155:195-200; & (b) Shanoudy H. Soliman A, Moe S, Hadian D, Veldhuis F, Iranmanesh A, Russell D, Early manifestations of "sick eythyroid syndrome" in patients with compensated chronic heart failure, J Card Fail 2001, 7(2):146-52; & (c)AE. Hak, HAP. Pols, TJ. Visser, et al., The Rotterdam Study., Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women, Ann Int Med, 2000, vol. 132, pp. 270--278 &(d)Thyroid Dysfunction Linked to Elevated Cardiac Risk, GSDL, http://www.gsdl.com/news/connections/vol12/conn20010411.html
&(e) Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med 2002 Dec 3;137(11):904-14; & (f)
B.G. Nedreboe, O. Nygard, et al, Plasma Total Homocysteine of hypothyroid patients during 12 months o
Integrated Medicine Dental Forum
http://www.DrEddyClinic.com/
http://dreddyclinic.com/forum/viewforum.php?f=4
DrEddyClinic.com links
- DrEddyClinic.com
- DrEddyClinic Alkaline Diet Blog
- pH miracle changes Living Alkalarian Health
- Dr. Eddy's Clinic Blog
- DrEddyClinic.com-project - Complementary and alternative medicine (CAM) Blog
- DrEddy'Clinic -Yeast/Fungus Blog
- Health News - Medicine, Diet, Fitness and Parenting from Health News Today
- DrEddyClinic.com - General Detoxification & Cleansing Blog
- DrEddyClinic.com - Live Blood Cell Darkfield Analyse Blog
- Integrative Medicine Blog
- Ayurveda School Blog
- Dr. Eddy Clinic & Ayurveda School
- Ayurveda Asia Co. Ltd.- Blog
- Dr. Eddy Clinic & Ayurveda School Blog
- Ayurveda in Thailand Blog
- Darkfield course
- Herbs for every day
- Today's Healthy Kitchen
- Anti Aging for Today
- Orthomolecular News Blog
Dr. Group's Secret to Health Kit$39.94  Dr. Group's Secret to Health Kit offers simple at-home solutions for cleansing internally and externally thereby reducing toxins, restoring the body's natural healing process, and helping you achieve true health and happiness. |
Advanced Body Cleansing Kit$147.75  Advanced Body Cleansing Kit with Livatrex™, Oxy-Powder®, Latero-Flora™ and two bottles of ParaTrex®. |